Korean J Obstet Gynecol.
2012 Sep;55(9):629-635. 10.5468/KJOG.2012.55.9.629.
Clostridium botulinum toxin A significantly inhibits uterine contractions in rats undergoing mifepristone-induced preterm labor
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Bundang CHA General Hospital, CHA University College of Medicine, Seongnam, Korea. bestob@cha.ac.kr
- KMID: 2274134
- DOI: http://doi.org/10.5468/KJOG.2012.55.9.629
Abstract
OBJECTIVE
We investigated the in vivo tocolytic effect of Clostridium botulinum toxin A (BoNT/A) on mifepristone-induced preterm labor in rats.
METHODS
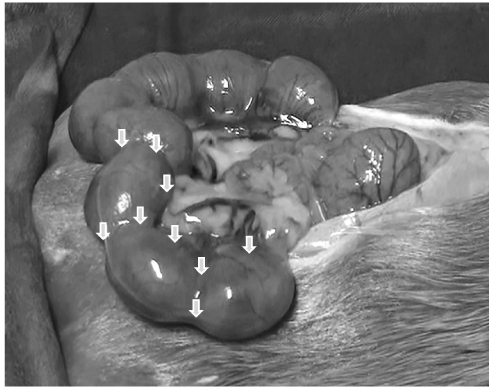
On day 17 of gestation, an incision was made to expose the uterus, and BoNT/A (normal saline or 20 units) was injected into the uterine horns. On day 18, mifepristone was used to induce uterine contractions. Electrical activity of uterine contractions was measured via electromyography on day 19.
RESULTS
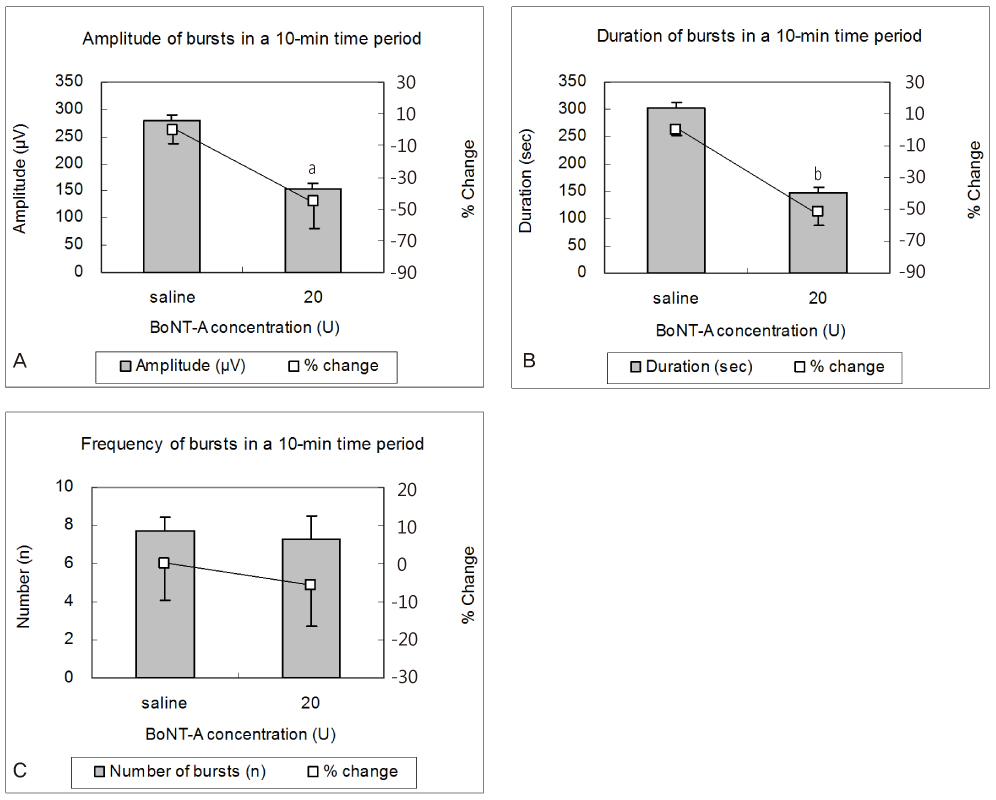
In rats treated with 20 units of BoNT/A, the amplitude of uterine contractions was significantly decreased by 45.2 +/- 18.4 (P<0.05) from baseline, respectively. The total duration of uterine contractions was significantly decreased by 51.7 +/- 7.9 (P<0.01), respectively. The frequency of contraction bursts after treatment with 20 units of BoNT/A was decreased by 5.6 +/- 16.3 from the baseline (P=0.4).
CONCLUSION
In rats undergoing mifepristone-induced preterm labor, BoNT/A significantly inhibited uterine contractility. The decrease in uterine activity was mainly caused by a decline in the duration and intensity rather than frequency of uterine contractions.
MeSH Terms
Figure
Reference
-
1. Wen SW, Smith G, Yang Q, Walker M. Epidemiology of preterm birth and neonatal outcome. Semin Fetal Neonatal Med. 2004. 9:429–435.2. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010. 88:31–38.3. Haas DM, Imperiale TF, Kirkpatrick PR, Klein RW, Zollinger TW, Golichowski AM. Tocolytic therapy: a meta-analysis and decision analysis. Obstet Gynecol. 2009. 113:585–594.4. Anotayanonth S, Subhedar NV, Garner P, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database Syst Rev. 2004. (4):CD004352.5. Jakuskiene R, Vollmer B, Saferis V, Daugeliene D. Neonatal outcomes of very preterm infants admitted to a tertiary center in Lithuania between the years 2003 and 2005. Eur J Pediatr. 2011. 170:1293–1303.6. Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000. 43:249–259.7. Brisinda G, Civello IM, Albanese A, Maria G. Gastrointestinal smooth muscles and sphincters spasms: treatment with botulinum neurotoxin. Curr Med Chem. 2003. 10:603–623.8. Karsenty G, Corcos J, Schurch B, Ruffion A, Chartier-Kastler E. Pharmacological treatment of neurogenic detrusor hyperactivity: intradetrusor botulinum toxin A injections. Prog Urol. 2007. 17:568–575.9. Garza JJ, Downard CD, Clayton N, Maher TJ, Fauza DO. Clostridium botulinum toxin inhibits myometrial activity in vitro: possible application on the prevention of preterm labor after fetal surgery. J Pediatr Surg. 2003. 38:511–513.10. Burd ID, Ness A, DiMuzio P, Ren GY, Tulenko TN. Clostridium botulinum toxin A inhibits contractility in pregnant human myometrium in vitro. Reprod Sci. 2009. 16:1001–1004.11. Doret M, Bukowski R, Longo M, Maul H, Maner WL, Garfield RE, et al. Uterine electromyography characteristics for early diagnosis of mifepristone-induced preterm labor. Obstet Gynecol. 2005. 105:822–830.12. Buhimschi C, Boyle MB, Saade GR, Garfield RE. Uterine activity during pregnancy and labor assessed by simultaneous recordings from the myometrium and abdominal surface in the rat. Am J Obstet Gynecol. 1998. 178:811–822.13. Morgan JC, Iyer SS, Moser ET, Singer C, Sethi KD. Botulinum toxin A during pregnancy: a survey of treating physicians. J Neurol Neurosurg Psychiatry. 2006. 77:117–119.14. Newman WJ, Davis TL, Padaliya BB, Covington CD, Gill CE, Abramovitch AI, et al. Botulinum toxin type A therapy during pregnancy. Mov Disord. 2004. 19:1384–1385.15. Duthie JB, Vincent M, Herbison GP, Wilson DI, Wilson D. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst Rev. 2011. (12):CD005493.16. Buhimschi CS, Buhimschi IA, Malinow AM, Saade GR, Garfield RE, Weiner CP. The forces of labour. Fetal Matern Med Rev. 2003. 14:273–307.17. Jin R, Sikorra S, Stegmann CM, Pich A, Binz T, Brunger AT. Structural and biochemical studies of botulinum neurotoxin serotype C1 light chain protease: implications for dual substrate specificity. Biochemistry. 2007. 46:10685–10693.18. Liu YL, Nwosu UC, Rice PJ. Relaxation of isolated human myometrial muscle by beta2-adrenergic receptors but not beta1-adrenergic receptors. Am J Obstet Gynecol. 1998. 179:895–898.19. Papka RE, Traurig HH, Schemann M, Collins J, Copelin T, Wilson K. Cholinergic neurons of the pelvic autonomic ganglia and uterus of the female rat: distribution of axons and presence of muscarinic receptors. Cell Tissue Res. 1999. 296:293–305.20. Dittrich R, Mueller A, Oppelt PG, Hoffmann I, Beckmann MW, Maltaris T. Differences in muscarinic-receptor agonist-, oxytocin-, and prostaglandin-induced uterine contractions. Fertil Steril. 2009. 92:1694–1700.21. Sand J, Nordback I, Arvola P, Pörsti I, Kalloo A, Pasricha P. Effects of botulinum toxin A on the sphincter of Oddi: an in vivo and in vitro study. Gut. 1998. 42:507–510.22. James AN, Ryan JP, Parkman HP. Inhibitory effects of botulinum toxin on pyloric and antral smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003. 285:G291–G297.23. Datta SN, Roosen A, Pullen A, Popat R, Rosenbaum TP, Elneil S, et al. Immunohistochemical expression of muscarinic receptors in the urothelium and suburothelium of neurogenic and idiopathic overactive human bladders, and changes with botulinum neurotoxin administration. J Urol. 2010. 184:2578–2585.24. Choppin A, Stepan GJ, Loury DN, Watson N, Eglen RM. Characterization of the muscarinic receptor in isolated uterus of sham operated and ovariectomized rats. Br J Pharmacol. 1999. 127:1551–1558.25. Kitazawa T, Hirama R, Masunaga K, Nakamura T, Asakawa K, Cao J, et al. Muscarinic receptor subtypes involved in carbachol-induced contraction of mouse uterine smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 2008. 377:503–513.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Mechanism of the Action of Clostridium botulinum Type B Neurotoxin
- A clinical study on the use of botulinum toxin type a in maxillofacial area
- Application of Botulinum Toxin Injection in Plastic Surgery
- Alternative Methods for Testing Botulinum Toxin: Current Status and Future Perspectives
- The First Outbreak of Botulism in Korea