Korean J Orthod.
2014 Nov;44(6):320-329. 10.4041/kjod.2014.44.6.320.
Compressive force regulates ephrinB2 and EphB4 in osteoblasts and osteoclasts contributing to alveolar bone resorption during experimental tooth movement
- Affiliations
-
- 1Department of Oral Pathology, School and Hospital of Stomatology, Jilin University, Changchun, China. hcsun@jlu.edu.cn
- 2Department of Orthodontics, School and Hospital of Stomatology, Jilin University, Changchun, China.
- 3Department of Endodontics, School and Hospital of Stomatology, Jilin University, Changchun, China.
- KMID: 2273232
- DOI: http://doi.org/10.4041/kjod.2014.44.6.320
Abstract
OBJECTIVE
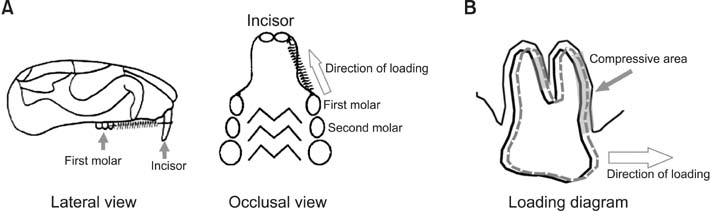
To investigate the involvement of ephrinB2 in periodontal tissue remodeling in compression areas during orthodontic tooth movement and the effects of compressive force on EphB4 and ephrinB2 expression in osteoblasts and osteoclasts.
METHODS
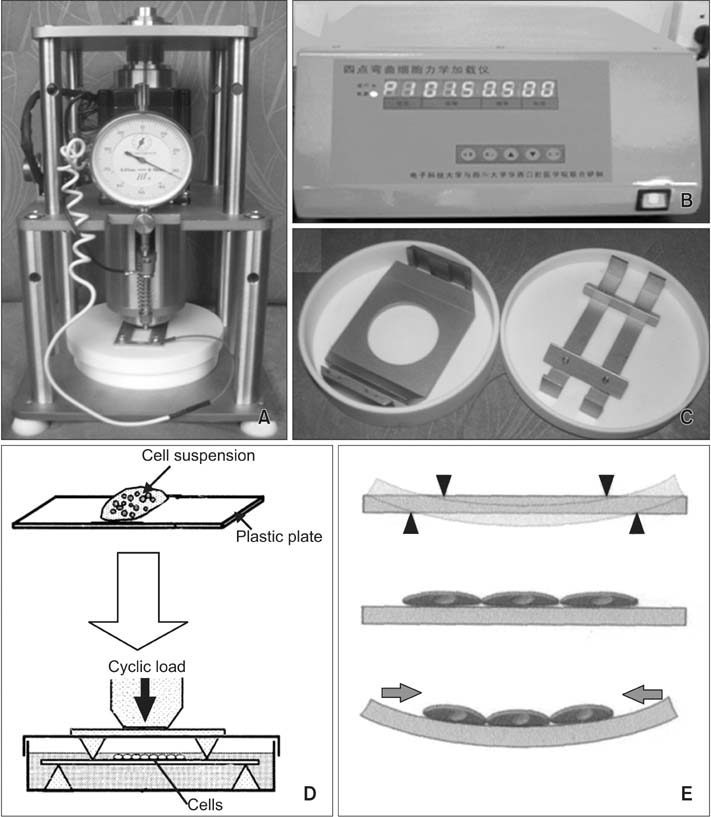
A rat model of experimental tooth movement was established to examine the histological changes and the localization of ephrinB2 in compressed periodontal tissues during experimental tooth movement. RAW264.7 cells and ST2 cells, used as precursor cells of osteoclasts and osteoblasts, respectively, were subjected to compressive force in vitro. The gene expression of EphB4 and ephrinB2, as well as bone-associated factors including Runx2, Sp7, NFATc1, and calcitonin receptor, were examined by quantitative real-time polymerase chain reaction (PCR).
RESULTS
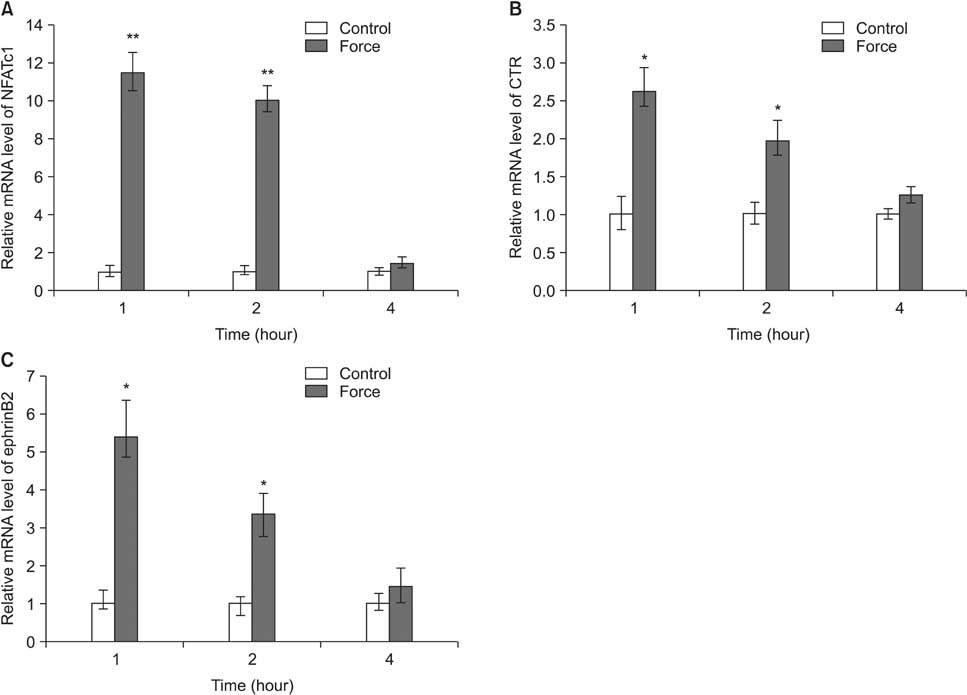
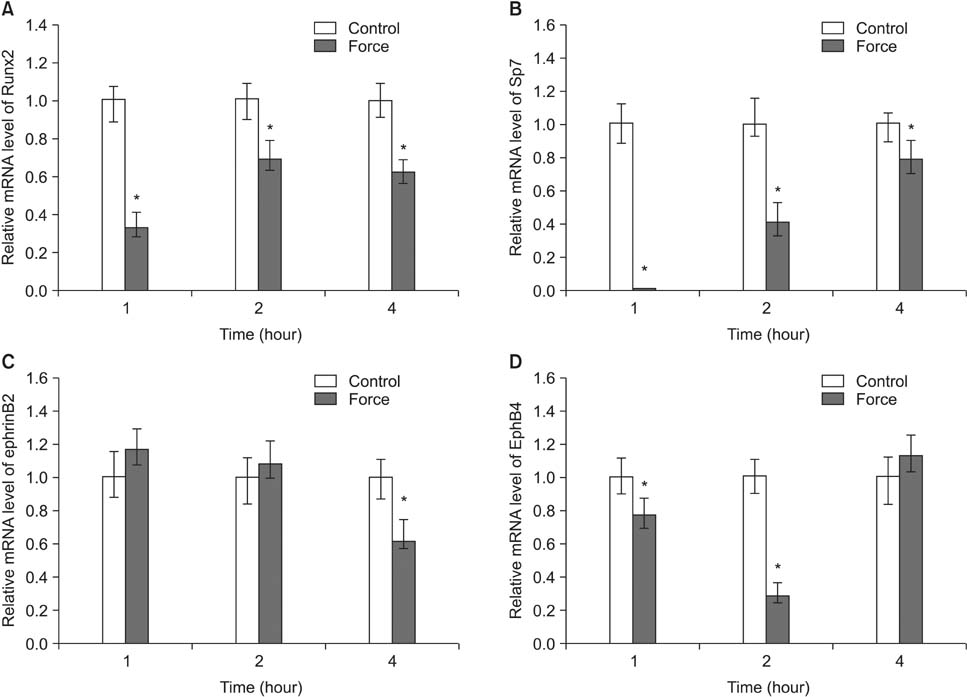
Histological examination of the compression areas of alveolar bone from experimental rats showed that osteoclastogenic activities were promoted while osteogenic activities were inhibited. Immunohistochemistry revealed that ephrinB2 was strongly expressed in osteoclasts in these areas. Quantitative real-time PCR showed that mRNA levels of NFATc1, calcitonin receptor, and ephrinB2 were increased significantly in compressed RAW264.7 cells, and the expression of ephrinB2, EphB4, Sp7, and Runx2 was decreased significantly in compressed ST2 cells.
CONCLUSIONS
Our results indicate that compressive force can regulate EphB4 and ephrinB2 expression in osteoblasts and osteoclasts, which might contribute to alveolar bone resorption in compression areas during orthodontic tooth movement.
Keyword
MeSH Terms
Figure
Reference
-
1. Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006; 129:469.e1–469.e32.
Article2. Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008; 87:414–434.
Article3. Masella RS, Meister M. Current concepts in the biology of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006; 129:458–468.
Article4. Roberts WE, Huja S, Roberts JA. Bone modeling: biomechanics, molecular mechanisms, and clinical perspectives. Sem Orthod. 2004; 10:123–161.
Article5. Oshiro T, Shiotani A, Shibasaki Y, Sasaki T. Osteoclast induction in periodontal tissue during experimental movement of incisors in osteoprotegerin-deficient mice. Anat Rec. 2002; 266:218–225.
Article6. Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002; 17:210–220.
Article7. Kanzaki H, Chiba M, Takahashi I, Haruyama N, Nishimura M, Mitani H. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res. 2004; 83:920–925.
Article8. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003; 423:349–355.
Article9. Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006; 4:111–121.
Article10. Edwards CM, Mundy GR. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int J Med Sci. 2008; 5:263–272.
Article11. Matsuo K. Eph and ephrin interactions in bone. Adv Exp Med Biol. 2010; 658:95–103.
Article12. Holder N, Durbin L, Cooke J. Eph receptors and ephrins are key regulators of morphogenesis. Ernst Schering Res Found Workshop. 2000; (29):123–147.
Article13. Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005; 6:462–475.
Article14. Allan EH, Häusler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008; 23:1170–1181.
Article15. Martin TJ, Allan EH, Ho PW, Gooi JH, Quinn JM, Gillespie MT, et al. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010; 658:51–60.
Article16. Obi S, Yamamoto K, Shimizu N, Kumagaya S, Masumura T, Sokabe T, et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol (1985). 2009; 106:203–211.
Article17. Diercke K, Kohl A, Lux CJ, Erber R. Strain-dependent up-regulation of ephrin-B2 protein in periodontal ligament fibroblasts contributes to osteogenesis during tooth movement. J Biol Chem. 2011; 286:37651–37664.
Article18. Diercke K, Sen S, Kohl A, Lux CJ, Erber R. Compression-dependent up-regulation of ephrin-A2 in PDL fibroblasts attenuates osteogenesis. J Dent Res. 2011; 90:1108–1115.
Article19. Suzuki N, Yoshimura Y, Deyama Y, Suzuki K, Kitagawa Y. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int J Mol Med. 2008; 21:291–296.
Article20. Liu J, Liu T, Zheng Y, Zhao Z, Liu Y, Cheng H, et al. Early responses of osteoblast-like cells to different mechanical signals through various signaling pathways. Biochem Biophys Res Commun. 2006; 348:1167–1173.
Article21. Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901.
Article22. Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005; 202:1261–1269.
Article23. Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med (Berl). 2005; 83:170–179.
Article24. Lee SK, Goldring SR, Lorenzo JA. Expression of the calcitonin receptor in bone marrow cell cultures and in bone: a specific marker of the differentiated osteoclast that is regulated by calcitonin. Endocrinology. 1995; 136:4572–4581.
Article25. Choi JY, Pratap J, Javed A, Zaidi SK, Xing L, Balint E, et al. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proc Natl Acad Sci U S A. 2001; 98:8650–8655.
Article26. Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, et al. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003; 63:5357–5362.27. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002; 108:17–29.
Article28. Kaback LA, Soung do Y, Naik A, Smith N, Schwarz EM, O'Keefe RJ, et al. Osterix/Sp7 regulates mesenchymal stem cell mediated endochondral ossification. J Cell Physiol. 2008; 214:173–182.
Article29. Waldo CM, Rothblatt JM. Histologic response to tooth movement in the laboratory rat; procedure and preliminary observations. J Dent Res. 1954; 33:481–486.
Article30. Zaki Ae, Vanhuysen G. Histology of the periodontium following tooth movement. J Dent Res. 1963; 42:1373–1379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An electron microscopic study on the alveolar bone remodelling in pressure zones of rat molar periodontium associated with orthodontic tooth movement
- Effects of bisphosphonate and indomethacin on alveolar bone remodeling in rats
- A histological and histochemical study on the periodontal tissue reaction during experimental tooth movement in the rat
- Mechanisms of Osteoclastogenesis in Orthodontic Tooth Movement and Orthodontically Induced Tooth Root Resorption
- Effect of vitamin C deficiency on the rate of orthodontic tooth movement and alveolar bone remodeling