Cancer Res Treat.
2006 Apr;38(2):99-107.
Protein Expression Profile using Two-Dimensional Gel Analysis in Squamous Cervical Cancer Patients
- Affiliations
-
- 1Cancer Research Institute, The Catholic University of Korea, Seoul, Korea.
- 2Department of Obstetrics and Gynecology, College of Medicine, Kyungpook National University, Daegu, Korea.
- 3Department of Obstetrics and Gynecology, College of Medicine, Soonchunhyang University, Asan, Korea.
- 4Department of Obstetrics and Gynecology, College of Medicine, Dankook University, Choenan, Korea.
- 5College of Pharmacy, Seoul National University, Seoul, Korea.
- 6KOMA Biotech, Seoul, Korea.
- 7Department of Bioscience and Biotechnology, Institute of Biotechnology, College of Life Science, Sejong University, Seoul, Korea.
- 8Department of Obstetrics and Gynecology, The Catholic University of Korea College of Medicine, Seoul, Korea. ahnws@catholic.ac.kr
Abstract
-
PURPOSE: Screening in cervical cancer is now progressing to discover candidate genes and proteins that may serve as biological markers and that play a role in tumor progression. We examined the protein expression patterns of the squamous cell carcinoma (SCC) tissues from Korean women with using two-dimensional polyacrylamide gel electrophoresis (2-DE) and matrix assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometer.
MATERIALS AND METHODS
Normal cervix and SCC tissues were solubilized and 2-DE was performed using pH 3~10 linear IPG strips of 17 cm length. The protein expression was evaluated using PDQuest 2-D software(TM). The differentially expressed protein spots were identified with a MALDI-TOF mass spectrometer, and the peptide mass spectra identifications were performed using the Mascot program and by searching the Swiss-prot or NCBInr databases.
RESULTS
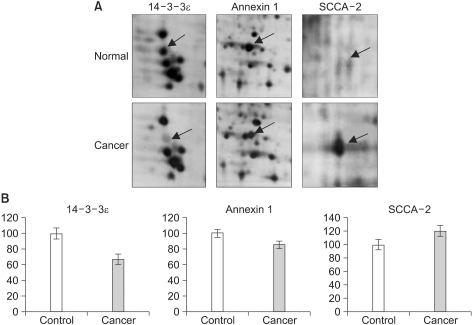
A total of 35 proteins were detected in SCC. 17 proteins were up-regulated and 18 proteins weredown-regulated. Among the proteins that were identified, 12 proteins (pigment epithelium derived factor, annexin A2 and A5, keratin 19 and 20, heat shock protein 27, smooth muscle protein 22 alpha, alpha-enolase, squamous cell carcinoma antigen 1 and 2, glutathione S-transferase and apolipoprotein a1) were protein previously known to be involved in tumor, and 21 proteins were newly identified in this study.
CONCLUSION
2-DE offers the total protein expression profiles of SCC tissues; further characterization of these differentially expressed proteins will give a chance to identify the badly needed tumor-specific diagnostic markers for SCC.
Keyword
MeSH Terms
-
Annexin A2
Apolipoproteins
Biomarkers
Carcinoma, Squamous Cell
Cervix Uteri
Databases, Protein
Electrophoresis, Polyacrylamide Gel
Epithelium
Female
Glutathione Transferase
HSP27 Heat-Shock Proteins
Humans
Hydrogen-Ion Concentration
Keratin-19
Mass Screening
Muscle, Smooth
Phosphopyruvate Hydratase
Uterine Cervical Neoplasms*
Annexin A2
Apolipoproteins
Glutathione Transferase
HSP27 Heat-Shock Proteins
Keratin-19
Phosphopyruvate Hydratase
Figure
Reference
-
1. Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002; 52:23–47. PMID: 11814064.
Article2. Kwon YI, Park TC, Park JS, Um SJ, Yu JH, Lee JM, et al. Accelerated induction of dyplastic lesion by TPA in HPV 18 URR E6/E7 gene expressing transgenic mice. J Korean Cancer Assoc. 2001; 33:56–63.3. zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002; 2:342–350. PMID: 12044010.
Article4. Monsonengo J, Bosch FX, Coursaget P, Cox JT, Franco E, Frazer I, et al. Cervical cancer control, priorities and new directions. Int J Cancer. 2004; 108:329–333. PMID: 14648697.5. Chen Y, Miller C, Mosher R, Zhao X, Deeds J, Morrissey M, et al. Identification of cervical cancer markers by cDNA and tissue microarrays. Cancer Res. 2003; 63:1927–1935. PMID: 12702585.6. Luo A, Kong J, Hu G, Liew CC, Xiong M, Wang X, et al. Discovery of Ca(2+)-relevant and differentiation-associated genes downregulated in esophageal squamous cell carcinoma using cDNA microarray. Oncogene. 2004; 23:1291–1299. PMID: 14647409.
Article7. Celis JE, Wolf H, Ostergaard M. Bladder squamous cell carcinoma biomarkers derived from proteomics. Electrophoresis. 2000; 21:2115–2121. PMID: 10892722.
Article8. Franzen B, Linder S, Alaiya AA, Eriksson E, Fujioka K, Bergman AC, et al. Analysis of polypeptide expression in benign and malignant human breast lesions. Electrophoresis. 1997; 18:582–587. PMID: 9150945.
Article9. Stulik J, Hernychova L, Porkertova S, Knizek J, Macela A, Bures J, et al. Proteome study of colorectal carcinogenesis. Electrophoresis. 2001; 22:3019–3025. PMID: 11565796.
Article10. Schmid HR, Schmitter D, Blum P, Miller M, Vonderschmitt D. Lung tumor cells: a multivariate approach to cell classification using two-dimensional protein pattern. Electrophoresis. 1995; 16:1961–1968. PMID: 8586071.
Article11. Hellman K, Alaiya AA, Schedvins K, Steinberg W, Hellstrom AC, Auer G. Protein expression patterns in primary carcinoma of the vagina. Br J Cancer. 2004; 91:319–326. PMID: 15199389.
Article12. Mortz E, Krogh TN, Vorum H, Gorg A. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics. 2001; 1:1359–1363. PMID: 11922595.
Article13. Ozaki T, Sakiyama S. Molecular cloning of rat calpactin I heavy-chain cDNA whose expression is induced in v-src-transformed rat culture cell lines. Oncogene. 1993; 8:1707–1710. PMID: 8389036.14. Alldridge LC, Bryant CE. Annexin 1 regulates cell proliferation by disruption of cell morphology and inhibition of cyclin D1 expression through sustained activation of the ERK1/2 MAPK signal. Exp Cell Res. 2003; 290:93–107. PMID: 14516791.
Article15. Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996; 84:889–897. PMID: 8601312.
Article16. Korneeva I, Bongiovanni AM, Girotra M, Caputo TA, Witkin SS. IgA antibodies to the 27-kDa heat-shock protein in the genital tracts of women with gynecologic cancers. Int J Cancer. 2000; 87:824–828. PMID: 10956393.
Article17. Lee KA, Shim JH, Kho CW, Park SG, Park BC, Kim JW, et al. Protein profiling and identification of modulators regulated by the E7 oncogene in the C33A cell line by proteomics and genomics. Proteomics. 2004; 4:839–848. PMID: 14997504.
Article18. Banks-Schlegel SP, Quintero J. Growth and differentiation of human esophageal carcinoma cell lines. Cancer Res. 1986; 46:250–258. PMID: 2415247.19. Pawlak G, McGarvey TW, Nguyen TB, Tomaszewski JE, Puthiyaveettil R, Malkowicz SB, et al. Alterations in tropomyosin isoform expression in human transitional cell carcinoma of the urinary bladder. Int J Cancer. 2004; 110:368–373. PMID: 15095301.
Article20. Shanahan CM, Weissberg PL, Metcalfe JC. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ Res. 1993; 73:193–204. PMID: 8508530.
Article21. Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977; 40:1621–1628. PMID: 332328.
Article22. Kato H, Suehiro Y, Morioka H, Torigoe T, Myoga A, Sekiguchi K, et al. Heterogeneous distribution of acidic TA-4 in cervical squamous cell carcinoma: immunohistochemical demonstration with monoclonal antibodies. Jpn J Cancer Res. 1987; 78:1246–1250. PMID: 3121560.23. Kato H, Nagaya T, Torigoe T. Heterogeneity of a tumor antigen TA-4 of squamous cell carcinoma in relation to its appearance in the circulation. Gann. 1984; 75:433–435. PMID: 6745563.24. McGettrick AF, Barnes RC, Worrall DM. SCCA2 inhibits TNF-mediated apoptosis in transfected HeLa cells: The reactive centre loop sequence is essential for this function and TNF-induced cathepsin G is a candidate target. Eur J Biochem. 2001; 268:5868–5875. PMID: 11722574.25. Suminami Y, Nagashima S, Murakami A, Nawata S, Gondo T, Hirakawa H, et al. Suppression of a squamous cell carcinoma (SCC)-related serpin, SCC antigen, inhibits tumor growth with increased intratumor infiltration of natural killer cells. Cancer Res. 2001; 61:1776–1780. PMID: 11280721.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Candidates for Tumor Markers of Cervical Cancer Discovered by Proteomic Analysis
- Plasma proteomic analysis of patients with squamous cell carcinoma of the uterine cervix
- Expression of Heat Shock Protein 60 kDa Is Upregulated in Cervical Cancer
- The Detection of the p53 Protein in Cervical Cancer and CIN by Immunohistochemistry
- TNF-alphaUp-regulated the Expression of HuR, a Prognostic Marker for Ovarian Cancer and Hu Syndrome, in BJAB Cells