Cancer Res Treat.
2006 Apr;38(2):78-83.
Efficacy of Combined Gemcitabine/Cisplatin Chemotherapy for Locally Advanced or Metastatic Urothelial Cancer
- Affiliations
-
- 1Department of Urology, School of Medicine, Kyung Hee University, Seoul, Korea. sgchang@khu.ac.kr
- 2Department of Urology, College of Medicine, Cheju National University, Jeju, Korea.
Abstract
-
PURPOSE: We wanted to determine and report on the outcome of combined gemcitabine/cisplatin chemotherapy for patients suffering with locally advanced or metastatic urothelial cancer.
MATERIALS AND METHODS
Between July 1999 and December 2004, 43 selected patients were enrolled in this study. Group 1 (the adjuvant chemotherapy group) had undergone radical surgery with removal of evident tumor from the following primary sites: bladder (n=8), renal pelvis (n=7) and ureter (n=3). Group 2 (the salvage chemotherapy group) had undergone palliative surgery with a remnant tumor at the following primary sites; bladder (n=23) and renal pelvis (n=2). All the patients were given gemcitabine/ciplatin and they evaluated for the therapeutic effect and toxicity. The patients were initially treated with gemcitabine 1000 mg/m2 intravenously for 30 minutes on days 1, 8 and 15 of a 28-day cycle, and cisplatin 70 mg/m2 was administered intravenously on day 1 using prehydration measures.
RESULTS
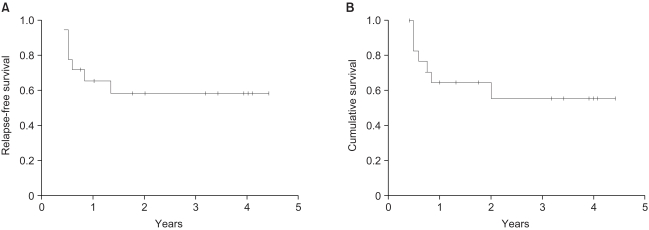
Group 1: The median follow-up period was 16.5 months. The mean age was 63 years (males: 15 cases, females: 3 cases), and eleven patients (61%) remained alive. The estimated median relapse-free survival period and 2-year survival rate were 24 months and 63%, respectively. Group 2: the median follow-up period was 20 months, the mean patient age was 63.8 years (males: 22 cases, females: 3 cases), and nine patients (36%) remained alive. The overall response and 2-year survival rates were 36% and 43%, respectively. Toxicities: Grade 3 toxicities developed in 14 cycles during the total 232 cycles. Grade 4 toxicity did not occur.
CONCLUSIONS
The results of this study confirm that adjuvant and salvage chemotherapy with using gemcitabine and cisplatin is tolerable and safe.
Keyword
MeSH Terms
Figure
Reference
-
1. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000; 18:3068–3077. PMID: 11001674.
Article2. Sternberg CN, Yagoda A, Scher HI, Watson RC, Ahmed T, Weiselberg LR, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol. 1985; 133:403–407. PMID: 4038749.
Article3. McCaffrey JA, Dodd PM, Herr H, Vlamis V, Mazumdar M, Higgins G, et al. Nonbladder primary site of transitional cell carcinoma (TCC) does not affect probability of response to M-VAC or survival (abstract 1299). Proc Am Soc Clin Oncol. 1998; 17:337a.4. Sternberg CN, Yagoda A, Scher HI, Watson RC, Geller N, Herr HW, et al. M-VAC for advanced transitional cell carcinoma of the urothelium: efficacy, and patterns of response and relapse. Cancer. 1989; 64:2448–2458. PMID: 2819654.5. Tannock I, Gospodarowicz M, Connolly J, Jewett M. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) chemotherapy for transitional cell carcinoma: the Princess Margaret Hospital experience. J Urol. 1989; 142(2 Pt 1):289–292. PMID: 2746745.
Article6. Saxman SB, Propert KJ, Einhorn LH, Crawford ED, Tannock I, Raghavan D, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1997; 15:2564–2569. PMID: 9215826.
Article7. Gitlitz BJ, Baker C, Chapman Y, Allen HJ, Bosserman LD, Patel R, et al. A phase II study of gemcitabine and docetaxel therapy in patients with advanced urothelial carcinoma. Cancer. 2003; 98:1863–1869. PMID: 14584068.
Article8. Calabro F, Sternberg CN. New drugs and new approaches for the treatment of metastatic urothelial cancer. World J Urol. 2002; 20:158–166. PMID: 12196899.
Article9. Bellmunt J, Albiol S. New chemotherapy combinations for advanced bladder cancer. Curr Opin Urol. 2001; 11:517–522. PMID: 11493774.
Article10. Lorusso V, Manzione L, De Vita F, Antimi M, Selvaggi FP, De Lena M. Gemcitabine plus cisplatin for advanced transitional cell carcinoma of the urinary tract: a phase II multicenter trial. J Urol. 2000; 164:53–56. PMID: 10840423.
Article11. Perry MC, Anderson CM, Donehower RC. Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, Mckenna WG, editors. Chemotherapy. Clinical oncology. 2004. New York: Churchill Livingstone;p. 492–493.12. Burch PA, Mailliard JA, Hillman DW, Perez EA, Krook JE, Rowland KM, et al. Phase II study of gemcitabine plus cisplatin in patients with metastatic breast cancer: a North Central Cancer Treatment Group Trial. Am J Clin Oncol. 2005; 28:195–200. PMID: 15803016.13. Wilkowski R, Thoma M, Duhmke E, Rau HG, Heinemann V. Concurrent chemoradiotherapy with gemcitabine and cisplatin after incomplete (R1) resection of locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2004; 58:768–772. PMID: 14967432.
Article14. Byrne MJ, Phillips M, Powell A, Cameron F, Joseph D, Spry N, et al. Cisplatin and gemcitabine induction chemotherapy followed by concurrent chemoradiotherapy or surgery for locally advanced non-small cell lung cancer. Intern Med J. 2005; 35:336–342. PMID: 15892762.
Article15. Segal R, Winquist E, Lukka H, Chin JL, Brundage M, Markman BR. Cancer Care Ontario Practice Guidelines Initiative Genitourinary Cancer Disease Site Group. Adjuvant chemotherapy for deep muscle-invasive transitional cell bladder carcinoma - a practice guideline. Can J Urol. 2002; 9:1625–1633. PMID: 12431323.16. Juffs HG, Moore MJ, Tannock IF. The role of systemic chemotherapy in the management of muscle-invasive bladder cancer. Lancet Oncol. 2002; 3:738–747. PMID: 12473515.
Article17. Suzuki S, Shinohara N, Harabayashi T, Sato S, Abe T, Koyanagi T. Impact of adjuvant systemic chemotherapy on postoperative survival in patients with high-risk urothelial cancer. Int J Urol. 2004; 11:456–460. PMID: 15242352.
Article18. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000; 18:3068–3077. PMID: 11001674.
Article19. Kaufman D, Raghavan D, Carducci M, Levine EG, Murphy B, Aisner J, et al. Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol. 2000; 18:1921–1927. PMID: 10784633.
Article20. Moore MJ, Winquist EW, Murray N, Tannock IF, Huan S, Bennett K, et al. Gemcitabine plus cisplatin, an active regimen in advanced urothelial cancer: a phase II trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1999; 17:2876–2881. PMID: 10561365.
Article21. Lehmann J, Retz M, Steiner G, Albers P, Jaeger E, Knuth A, et al. Gemcitabine/cisplatin vs MVAC 5 year survival outcome of the phase III study of chemotherapy of advanced urothelial carcinoma in Germany. Urologe A. 2003; 42:1074–1086. PMID: 14513232.22. de Wit R, Bellmunt J. Overview of gemcitabine triplets in metastatic bladder cancer. Crit Rev Oncol Hematol. 2003; 45:191–197. PMID: 12604129.23. Meliani E, Lapini A, Serni S, Corvino C, Carini M. Gemcitabine plus cisplatin in adjuvant regimen for bladder cancer. Toxicity evalu ation. Urol Int. 2003; 71:37–40. PMID: 12845258.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gemcitabine versus Gemcitabine Combined with Cisplatin Treatment Locally Advanced or Metastatic Pancreatic Cancer: A Retrospective Analysis
- Efficacy and Toxicity of Gemcitabine Based Chemotherapy for Advanced Urothelial Cancer
- Long Term Complete Response of Unresectable Locally Advanced Pancreatic Cancer after CCRT and Gemcitabine Chemotherapy
- Three-Weekly Gemcitabine Plus Cisplatin Chemotherapy in Patients with Locally Advanced or Metastatic Non-small-cell Lung Cancer: Phase II Study of the Korean Association for the Study of Lung Cancer
- Chemotherapy in Advanced Urothelial Carcinoma