Blood Res.
2013 Mar;48(1):16-23. 10.5045/br.2013.48.1.16.
Expression of SOCS1 and SOCS3 genes in human graft-versus-host disease after allogeneic hematopoietic stem cell transplantation
- Affiliations
-
- 1Division of Hematology, Department of Internal Medicine, Catholic Blood and Marrow Transplantation Center, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. cumckim@catholic.ac.kr
- KMID: 2270721
- DOI: http://doi.org/10.5045/br.2013.48.1.16
Abstract
- BACKGROUND
Suppressor of cytokine signaling genes (SOCS) are regarded as pivotal negative feedback regulators of cytokine signals, including the interferon-gamma (IFN-gamma), granulocyte-colony stimulating factor, and interleukin families, released by T cells. A detailed understanding of the involvement of SOCS genes in graft-versus-host disease (GVHD) is critical to effectively manage GVHD, yet their expression patterns among recipients remain largely unexplored.
METHODS
Expression levels of SOCS1 and SOCS3 were determined by real-time quantitative reverse transcription PCR (qRT-PCR) in patients with acute GVHD (aGVHD) and chronic GVHD (cGVHD), in a severity-dependent manner, after allogeneic hematopoietic stem cell transplantation (HSCT). A total of 71 recipients with AML (N=40), ALL (N=12), myelodysplastic syndromes (MDS; N=10), chronic myelogenous leukemia (CML; N=2), severe aplastic anemia (SAA; N=5), or others (N=2), who received allogeneic HSCT from human leukocyte antigen-identical siblings or unrelated donors between 2009 and 2011, were included in the present study.
RESULTS
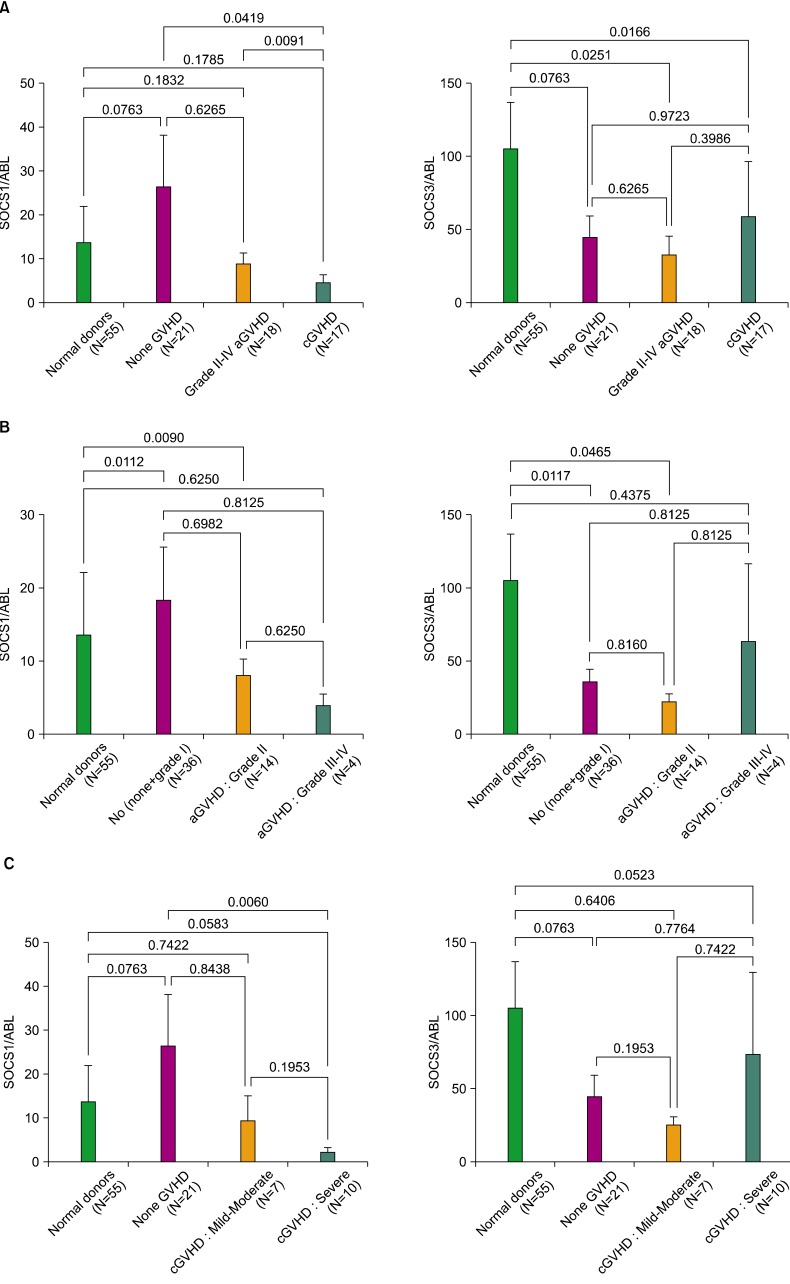
Overall, the expression levels of SOCS1 decreased in recipients with grade II to IV aGVHD and cGVHD when compared to normal donors and non-GVHD recipients. Interestingly, the expressions of SOCS1 decreased significantly more in cGVHD than in aGVHD recipients (P=0.0091). In contrast, SOCS3 expressions were similarly reduced in all the recipients.
CONCLUSION
This is the first study to show that SOCS1 and SOCS3 are differentially expressed in recipients following allogeneic HSCT, suggesting a prognostic correlation between SOCS genes and the development of GVHD. This result provides a new platform to study GVHD immunobiology and potential diagnostic and therapeutic targets for GVHD.
Keyword
MeSH Terms
-
Anemia, Aplastic
Graft vs Host Disease
Hematopoietic Stem Cell Transplantation
Hematopoietic Stem Cells
Humans
Interferon-gamma
Interleukins
Leukemia, Myelogenous, Chronic, BCR-ABL Positive
Leukocytes
Myelodysplastic Syndromes
Polymerase Chain Reaction
Real-Time Polymerase Chain Reaction
Reverse Transcription
Siblings
Suppressor of Cytokine Signaling Proteins
T-Lymphocytes
Tissue Donors
Transplantation, Homologous
Unrelated Donors
Interferon-gamma
Interleukins
Suppressor of Cytokine Signaling Proteins
Figure
Reference
-
1. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009; 373:1550–1561. PMID: 19282026.
Article2. Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012; 12:443–458. PMID: 22576252.
Article3. Koyama M, Kuns RD, Olver SD, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat Med. 2011; 18:135–142. PMID: 22127134.
Article4. Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010; 30:75–101. PMID: 20113888.
Article5. Min CK. The pathophysiology of chronic graft-versus-host disease: the unveiling of an enigma. Korean J Hematol. 2011; 46:80–87. PMID: 21747879.
Article6. Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000; 96:2062–2068. PMID: 10979948.7. Highfill SL, Rodriguez PC, Zhou Q, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010; 116:5738–5747. PMID: 20807889.
Article8. Nishimori H, Maeda Y, Teshima T, et al. Synthetic retinoid Am80 ameliorates chronic graft-versus-host disease by down-regulating Th1 and Th17. Blood. 2012; 119:285–295. PMID: 22077062.
Article9. Ichiba T, Teshima T, Kuick R, et al. Early changes in gene expression profiles of hepatic GVHD uncovered by oligonucleotide microarrays. Blood. 2003; 102:763–771. PMID: 12663442.
Article10. Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002; 99:3493–3499. PMID: 11986199.
Article11. Antin JH. T-cell depletion in GVHD: less is more? Blood. 2011; 117:6061–6062. PMID: 21659553.
Article12. Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000; 105:1289–1298. PMID: 10792004.
Article13. Yi T, Chen Y, Wang L, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009; 114:3101–3112. PMID: 19602708.
Article14. Matsumoto A, Seki Y, Watanabe R, et al. A role of suppressor of cytokine signaling 3 (SOCS3/CIS3/SSI3) in CD28-mediated interleukin 2 production. J Exp Med. 2003; 197:425–436. PMID: 12591901.
Article15. Croker BA, Krebs DL, Zhang JG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003; 4:540–545. PMID: 12754505.
Article16. Naka T, Fujimoto M, Tsutsui H, Yoshimura A. Negative regulation of cytokine and TLR signalings by SOCS and others. Adv Immunol. 2005; 87:61–122. PMID: 16102572.
Article17. Heeg K, Dalpke A. TLR-induced negative regulatory circuits: role of suppressor of cytokine signaling (SOCS) proteins in innate immunity. Vaccine. 2003; 21(Suppl 2):S61–S67. PMID: 12763685.
Article18. Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003; 4:1169–1176. PMID: 14639467.
Article19. Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol. 2002; 2:410–416. PMID: 12093007.
Article20. Alexander WS, Starr R, Fenner JE, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999; 98:597–608. PMID: 10490099.21. Hill GR, Kuns RD, Raffelt NC, et al. SOCS3 regulates graft-versus-host disease. Blood. 2010; 116:287–296. PMID: 20435883.
Article22. Johan MF, Bowen DT, Frew ME, Goodeve AC, Reilly JT. Aberrant methylation of the negative regulators RASSFIA, SHP-1 and SOCS-1 in myelodysplastic syndromes and acute myeloid leukaemia. Br J Haematol. 2005; 129:60–65. PMID: 15801956.
Article23. Reddy PN, Sargin B, Choudhary C, et al. SOCS1 cooperates with FLT3-ITD in the development of myeloproliferative disease by promoting the escape from external cytokine control. Blood. 2012; 120:1691–1702. PMID: 22517899.
Article24. Yasukawa H, Yajima T, Duplain H, et al. The suppressor of cytokine signaling-1 (SOCS1) is a novel therapeutic target for enterovirus-induced cardiac injury. J Clin Invest. 2003; 111:469–478. PMID: 12588885.
Article25. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995; 15:825–828. PMID: 7581076.26. Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003; 9:215–233. PMID: 12720215.
Article27. Cho BS, Yoon JH, Shin SH, et al. Comparison of allogeneic stem cell transplantation from familial-mismatched/haploidentical donors and from unrelated donors in adults with high-risk acute myelogenous leukemia. Biol Blood Marrow Transplant. 2012; 18:1552–1563. PMID: 22516055.
Article28. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005; 11:945–956. PMID: 16338616.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of pneumomediastinum combined with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation
- SOCS1 and SOCS3 are expressed in mononuclear cells in human cytomegalovirus viremia after allogeneic hematopoietic stem cell transplantation
- Chronic graft versus host disease with small bowel obstruction after unrelated hematopoietic stem cell transplantation in a patient with acute myeloid leukemia
- Eosinophilic Fasciitis as the Initial Manifestation of Graft-Versus-Host Disease
- Graft-versus-Leukemia Effect of Nonmyeloablative Stem Cell Transplantation