Korean J Nutr.
2012 Jun;45(3):211-217. 10.4163/kjn.2012.45.3.211.
Dietary effect of red ginseng extracts mixed with torilis fructus and corni fructus on the epidermal levels of ceramides and ceramide related enzyme proteins in uv-induced hairless mice
- Affiliations
-
- 1Department of Medical Nutrition, Graduate School of East-West Medical Science, Kyung Hee University, Yongin 446-701, Korea. choyunhi@khu.ac.kr
- KMID: 2268685
- DOI: http://doi.org/10.4163/kjn.2012.45.3.211
Abstract
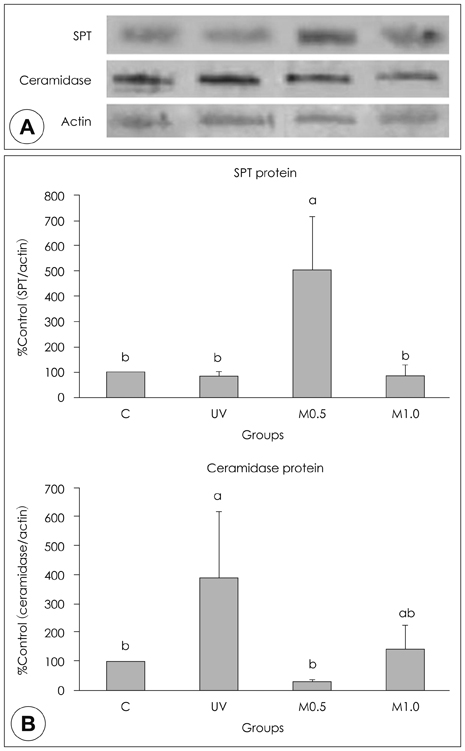
- UV-irradiation is a major factor of photo-aged skin, by which pigmentation, wrinkles and laxity are increased. In addition, the epidermal barrier is disrupted, ultimately causing dryness in photo-aged skin. As an effort to search dietary sources for improving the dryness of UV irradiated skin, the dietary effect of red ginseng based functional foods on the epidermal level of ceramides, a major lipid maintaining epidermal barrier, was determined in this study. Albino hairless mice were fed either a control diet [group UV (UV-irradiated control)] or diets with 0.5% (group M0.5) or 1% (group M1.0) of red ginseng extracts mixed with Torilis fructus and Corni fructus (66.7% red ginseng) in parallel with UV irradiation for 5 wks. A normal control group (group C) was fed a control diet without UV irradiation for 5 wks. The epidermal level of ceramides in group UV was significantly lower than that in group C, in which ceramidase, an enzyme involved in ceramide degradation, was highly expressed. In group M0.5, the epidermal level of ceramide was significantly increased to the level even higher than in group C. In addition, protein expression of serine palmitoyl transferase (SPT), a key enzyme involved in de novo ceramide synthesis, was increased in group M0.5. However the epidermal levels of ceramides as well as of ceramidase protein expression in group M1.0 did not differ from those in group UV. In conclusion, we demonstrate that dietary supplementation of red-ginseng extracts mixed with Torilis fructus and Corni fructus at a level of 0.5% level in diet increased the epidermal level of ceramides coupled with the elevated expression of SPT protein.
Keyword
MeSH Terms
Figure
Reference
-
1. Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983. 80:Suppl 1. 44s–49s.
Article2. Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007. 127(7):1574–1576.
Article3. Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991. 24:1–26.
Article4. Harding CR. The stratum corneum: structure and function in health and disease. Dermatol Ther. 2004. 17:Suppl 1. 6–15.
Article5. Holleran WM, Takagi Y, Imokawa G, Jackson S, Lee JM, Elias PM. beta-Glucocerebrosidase activity in murine epidermis: characterization and localization in relation to differentiation. J Lipid Res. 1992. 33(8):1201–1209.
Article6. Takagi Y, Kriehuber E, Imokawa G, Elias PM, Holleran WM. Beta-glucocerebrosidase activity in mammalian stratum corneum. J Lipid Res. 1999. 40(5):861–869.7. Dickson RC, Lester RL, Nagiec MM. Serine palmitoyltransferase. Methods Enzymol. 2000. 311:3–9.8. Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. 2003. 1632(1-3):16–30.
Article9. Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002. 1(4):705–720.
Article10. Meguro S, Arai Y, Masukawa Y, Uie K, Tokimitsu I. Relationship between covalently bound ceramides and transepidermal water loss (TEWL). Arch Dermatol Res. 2000. 292(9):463–468.
Article11. Meguro S, Arai Y, Masukawa K, Uie K, Tokimitsu I. Stratum corneum lipid abnormalities in UVB-irradiated skin. Photochem Photobiol. 1999. 69(3):317–321.
Article12. Menon GK, Ghadially R, Williams ML, Elias PM. Lamellar bodies as delivery systems of hydrolytic enzymes: implications for normal and abnormal desquamation. Br J Dermatol. 1992. 126(4):337–345.
Article13. Kim H, Oh I, Park KH, Kim NM, Do JH, Cho Y. Stimulatory effect of dietary red ginseng on epidermal hydration and ceramide levels in ultraviolet-irradiated hairless mice. J Med Food. 2009. 12(4):746–754.
Article14. Bak H, Hong SP, Jeong SK, Choi EH, Lee SE, Lee SH, Ahn SK. Altered epidermal lipid layers induced by long-term exposure to suberythemal-dose ultraviolet. Int J Dermatol. 2011. 50(7):832–837.
Article15. Boelsma E, Hendriks HF, Roza L. Nutritional skin care: health effects of micronutrients and fatty acids. Am J Clin Nutr. 2001. 73(5):853–864.
Article16. Smith M, Boon HS. Counseling cancer patients about herbal medicine. Patient Educ Couns. 1999. 38(2):109–120.
Article17. Song JH, Park MJ, Kim E, Kim YC. Effect of Panax ginseng on galactosamine-induced cytotoxicity in primary cultured rat hepatocytes. Yakhak Hoeji. 1990. 34(5):341–347.18. Tomoda M, Takeda K, Shimizu N, Gonda R, Ohara N, Takada K, Hirabayashi K. Characterization of two acidic polysaccharides having immunological activities from the root of Panax ginseng. Biol Pharm Bull. 1993. 16(1):22–25.
Article19. Park JD. Recent studies on the chemical constituents of Korean ginseng (Panax ginseng C. A. Meyer). Korean J Ginseng Sci. 1996. 20(4):389–415.20. Kwak YS, Park JD, Yang JW. Present and its prospect of red ginseng efficacy research. Food Ind Nutr. 2003. 8(2):30–37.21. Nam KY. The comparative understanding between red ginseng and white ginsengs, processed ginsengs (Panax ginseng C. A. Meyer). J Ginseng Res. 2005. 29(1):1–18.
Article22. Zhang D, Yasuda T, Yu Y, Zheng P, Kawabata T, Ma Y, Okada S. Ginseng extract scavenges hydroxyl radical and protects unsaturated fatty acids from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med. 1996. 20(1):145–150.
Article23. An DK. Medicinal herbs. 2003. Seoul: Kyohak.24. Shin JH, Shin YW. Illustrated book of medical herbs from hyangyakjipseongbang. 2006. Daegu: Keimyung University Press.25. Lee EB, Choi BC, Cho TS. Pharmacological studies on ether fraction of Corni fructus. Yakhak Hoeji. 1985. 29(1):1–10.26. Park JH. Medical plants of Korea. 2004. Seoul: Shinil books.27. Choi WY, Chun HJ, Lee JH, Baek SH. Effects of methanol extract from Cornis fructus on melanogenesis. Korean J Pharmacogn. 2003. 34(1):70–74.28. Cha HJ, Park MT, Chung HY, Kim ND, Sato H, Seiki M, Kim KW. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene. 1998. 16(6):771–778.
Article29. So SH, Lee SK, Hwang EI, Koo BS, Han GH, Kim NM. Effects of Korean red ginseng and herb extracts mixture (KTNG0345) on procollagen biosynthesis and matrix metalloproteinase-1 activity in human dermal fibroblast. J Ginseng Res. 2007. 31(4):196–202.
Article30. Park KH, Choi YS, Kim HA, Lee KG, Yeo JH, Jung DH, Kim SH, Cho Y. Dietary effect of silk protein on ceramide synthesis and the expression of ceramide metabolic enzymes in the epidermis of NC/Nga mice. J Korean Soc Food Sci Nutr. 2007. 36(5):554–562.
Article31. Tohyama J, Oya Y, Ezoe T, Vanier MT, Nakayasu H, Fujita N, Suzuki K. Ceramide accumulation is associated with increased apoptotic cell death in cultured fibroblasts of sphingolipid activator protein-deficient mouse but not in fibroblasts of patients with Farber disease. J Inherit Metab Dis. 1999. 22(5):649–662.
Article32. McAuliffe DJ, Blank IH. Effects of UVA (320-400 nm) on the barrier characteristics of the skin. J Invest Dermatol. 1991. 96(5):758–762.
Article33. Elias PM, Friend DS. The permeability barrier in mammalian epidermis. J Cell Biol. 1975. 65(1):180–191.
Article34. Ohnishi Y, Okino N, Ito M, Imayama S. Ceramidase activity in bacterial skin flora as a possible cause of ceramide deficiency in atopic dermatitis. Clin Diagn Lab Immunol. 1999. 6(1):101–104.
Article35. Imokawa G, Takema Y, Yorimoto Y, Tsukahara K, Kawai M, Imayama S. Degree of ultraviolet-induced tortuosity of elastic fibers in rat skin is age dependent. J Invest Dermatol. 1995. 105(2):254–258.
Article36. Holleran WM, Uchida Y, Halkier-Sorensen L, Haratake A, Hara M, Epstein JH, Elias PM. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol Photoimmunol Photomed. 1997. 13(4):117–128.
Article37. Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006. 580(23):5456–5466.
Article38. Uchida Y, Nardo AD, Collins V, Elias PM, Holleran WM. De novo ceramide synthesis participates in the ultraviolet B irradiation-induced apoptosis in undifferentiated cultured human keratinocytes. J Invest Dermatol. 2003. 120(4):662–669.
Article39. Kang KS, Kim HY, Pyo JS, Yokozawa T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol Pharm Bull. 2006. 29(4):750–754.
Article40. Halladay AK, Yu YL, Palmer J, Oh KW, Wagner GC. Acute and chronic effects of ginseng total saponin and amphetamine on fixed-interval performance in rats. Planta Med. 1999. 65(2):162–164.
Article41. Bae EA, Han MJ, Shin YW, Kim DH. Inhibitory effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol Pharm Bull. 2006. 29(9):1862–1867.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antidiabetic Effects of Corni Fructus Extract in Streptozotocin-Induced Diabetic Rats
- Dietary effect of green tea extract on hydration improvement and metabolism of free amino acid generation in epidermis of UV-irradiated hairless mice
- Corni Fructus-Induced Acute Interstitial Nephritis
- Arctii Fructus is a Prominent Dietary Source of Linoleic Acid for Reversing Epidermal Hyperproliferation of Guinea Pigs
- Dietary effect of royal jelly supplementation on epidermal levels of hydration, filaggrins, free amino acids and the related enzyme expression in UV irradiated hairless mice