Ann Dermatol.
2010 Nov;22(4):379-388. 10.5021/ad.2010.22.4.379.
Cholesterol, a Major Component of Caveolae, Down-regulates Matrix Metalloproteinase-1 Expression through ERK/JNK Pathway in Cultured Human Dermal Fibroblasts
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Dermatology, Seoul National University College of Medicine, and Laboratory of Cutaneous Aging Research, Clinical Research Institute, Seoul National University Hospital, and Institute of Dermatological Science, Seoul National University, Seou
- KMID: 2266173
- DOI: http://doi.org/10.5021/ad.2010.22.4.379
Abstract
- BACKGROUND
Cholesterol is a major component of specialized membrane microdomains known as lipid rafts or caveolae, which modulate the fluidity of biological membranes. Membrane cholesterol therefore plays an important role in cell signaling and vesicular transport.
OBJECTIVE
In this study, we investigated the effects of cholesterol on matrix metalloproteinase-1 (MMP-1) expression in human dermal fibroblasts.
METHODS
MMP-1 mRNA and protein expression were determined by RT-PCR and Western blotting, respectively. AP-1 DNA binding activity was detected by electrophoretic mobility shift assays. The amount of cholesterol was analyzed by cholesterol assay kit.
RESULTS
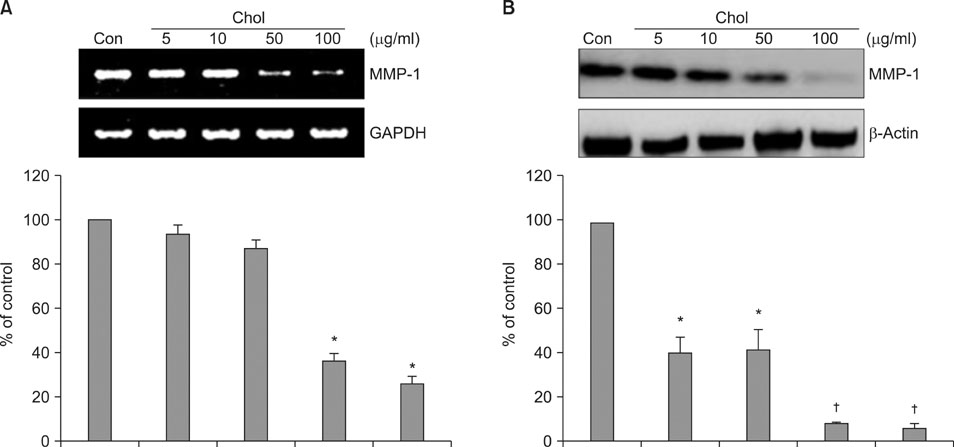
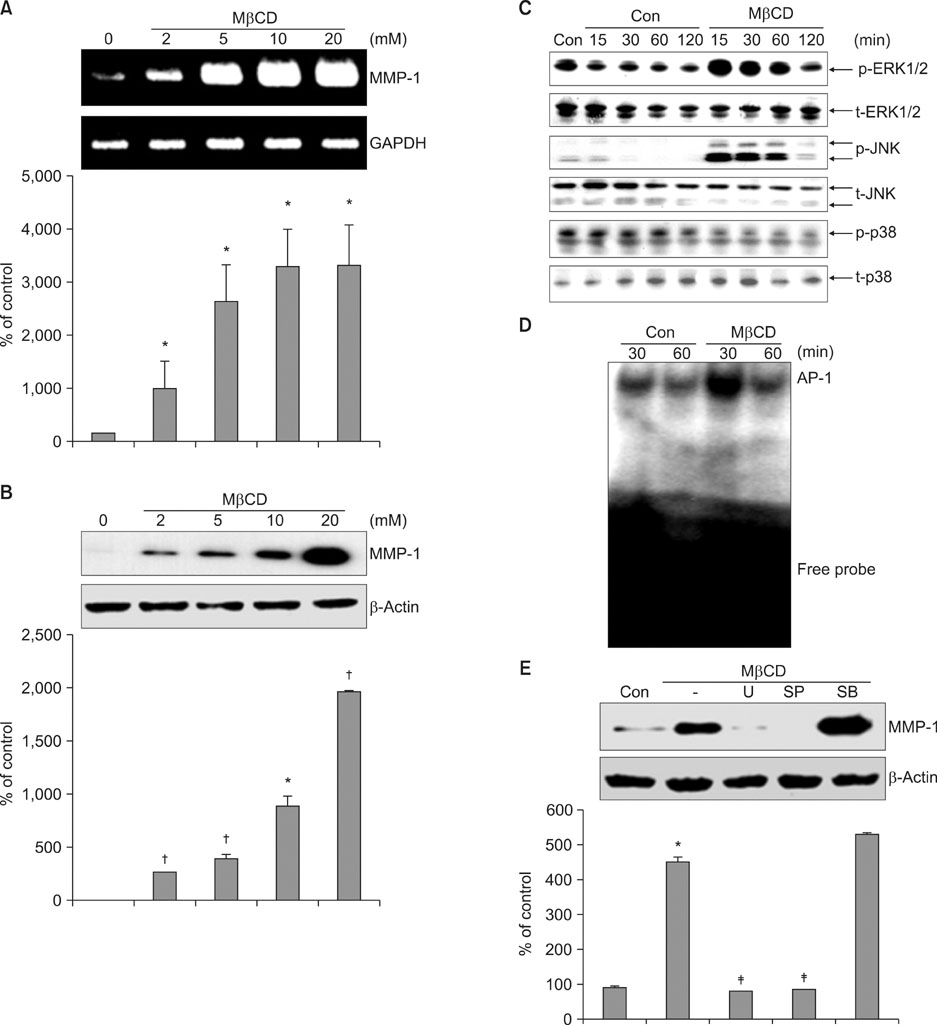
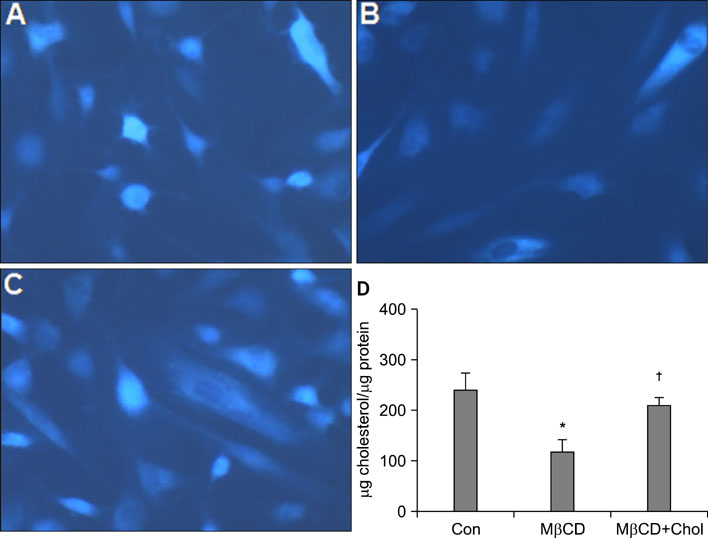
We observed that MMP-1 mRNA and protein expression was dose-dependently decreased by cholesterol treatment. In contrast, cholesterol depletion by a cholesterol depletion agent, methyl-beta-cyclodextrin (M beta CD) in human dermal fibroblasts, increased MMP-1 mRNA and protein expression in a dose-dependent manner. Also, we investigated the regulatory mechanism of M beta CD-induced MMP-1 expression: cholesterol depletion by M beta CD, activated ERK1/2 and JNK, but not p38 MAPK cascade, and it also significantly increased c-Jun phosphorylation, c-Fos expression and activator protein-1 binding activity. Furthermore, the inhibition of ERK or JNK with specific chemical inhibitors prevented M beta CD-induced MMP-1 expression, which indicates that ERK and JNK play an important role in cholesterol depletion-mediated MMP-1 induction. In addition, M beta CD-induced phosphorylation of ERK and JNK and MMP-1 expression were suppressed by cholesterol repletion.
CONCLUSION
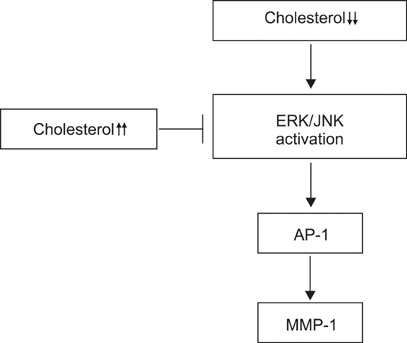
Our results suggest that cholesterol regulates MMP-1 expression through the control of ERK and JNK activity in human dermal fibroblasts.
Keyword
MeSH Terms
-
beta-Cyclodextrins
Blotting, Western
Caveolae
Cholesterol
DNA
Electrophoretic Mobility Shift Assay
Fibroblasts
Humans
Matrix Metalloproteinase 1
Membrane Microdomains
Membranes
p38 Mitogen-Activated Protein Kinases
Phosphorylation
RNA, Messenger
Transcription Factor AP-1
Cholesterol
DNA
Matrix Metalloproteinase 1
RNA, Messenger
Transcription Factor AP-1
beta-Cyclodextrins
p38 Mitogen-Activated Protein Kinases
Figure
Cited by 1 articles
-
Cholesterol Depletion in Cell Membranes of Human Airway Epithelial Cells Suppresses MUC5AC Gene Expression
Kee Jae Song, Na Hyun Kim, Gi Bong Lee, Ji Hoon Kim, Jin Ho Kwon, Kyung-Su Kim
Yonsei Med J. 2013;54(3):679-685. doi: 10.3349/ymj.2013.54.3.679.
Reference
-
1. Grubauer G, Feingold KR, Elias PM. Relationship of epidermal lipogenesis to cutaneous barrier function. J Lipid Res. 1987. 28:746–752.
Article2. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990. 343:425–430.
Article3. Schmidt R, Parish EJ, Dionisius V, Cathelineau C, Michel S, Shroot B, et al. Modulation of cellular cholesterol and its effect on cornified envelope formation in cultured human epidermal keratinocytes. J Invest Dermatol. 1991. 97:771–775.
Article4. Demel RA, De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976. 457:109–132.
Article5. Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000. 1:31–39.
Article6. Pike LJ, Casey L. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry. 2002. 41:10315–10322.
Article7. Alvarez E, Ruiz-Gutiérrez V, Santa María C, Machado A. Age-dependent modification of lipid composition and lipid structural order parameter of rat peritoneal macrophage membranes. Mech Ageing Dev. 1993. 71:1–12.
Article8. Cohen BM, Zubenko GS. Aging and the biophysical properties of cell membranes. Life Sci. 1985. 37:1403–1409.
Article9. Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000. 275:17221–17224.
Article10. Ringerike T, Blystad FD, Levy FO, Madshus IH, Stang E. Cholesterol is important in control of EGF receptor kinase activity but EGF receptors are not concentrated in caveolae. J Cell Sci. 2002. 115:1331–1340.
Article11. McGuire TF, Corey SJ, Sebti SM. Lovastatin inhibits platelet-derived growth factor (PDGF) stimulation of phosphatidylinositol 3-kinase activity as well as association of p85 subunit to tyrosine-phosphorylated PDGF receptor. J Biol Chem. 1993. 268:22227–22230.
Article12. Schönfelder U, Radestock A, Elsner P, Hipler UC. Cyclodextrin-induced apoptosis in human keratinocytes is caspase-8 dependent and accompanied by mitochondrial cytochrome c release. Exp Dermatol. 2006. 15:883–890.
Article13. Kerkelä E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003. 12:109–125.
Article14. Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002. 1:705–720.
Article15. Lee J, Jung E, Lee J, Huh S, Hwang CH, Lee HY, et al. Emodin inhibits TNF alpha-induced MMP-1 expression through suppression of activator protein-1 (AP-1). Life Sci. 2006. 79:2480–2485.
Article16. Maldonado A, Game BA, Song L, Huang Y. Up-regulation of matrix metalloproteinase-1 expression in U937 cells by low-density lipoprotein-containing immune complexes requires the activator protein-1 and the Ets motifs in the distal and the proximal promoter regions. Immunology. 2003. 109:572–579.
Article17. Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989. 109:877–889.
Article18. Przybylowska K, Kluczna A, Zadrozny M, Krawczyk T, Kulig A, Rykala J, et al. Polymorphisms of the promoter regions of matrix metalloproteinases genes MMP-1 and MMP-9 in breast cancer. Breast Cancer Res Treat. 2006. 95:65–72.
Article19. Doyle GA, Pierce RA, Parks WC. Transcriptional induction of collagenase-1 in differentiated monocyte-like (U937) cells is regulated by AP-1 and an upstream C/EBP-beta site. J Biol Chem. 1997. 272:11840–11849.
Article20. Reunanen N, Westermarck J, Häkkinen L, Holmström TH, Elo I, Eriksson JE, et al. Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J Biol Chem. 1998. 273:5137–5145.
Article21. Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen-activated protein kinase by SB 203580 blocks PMA-induced Mr 92,000 type IV collagenase secretion and in vitro invasion. Cancer Res. 1998. 58:1135–1139.22. Simon C, Simon M, Vucelic G, Hicks MJ, Plinkert PK, Koitschev A, et al. The p38 SAPK pathway regulates the expression of the MMP-9 collagenase via AP-1-dependent promoter activation. Exp Cell Res. 2001. 271:344–355.
Article23. Arnott CH, Scott KA, Moore RJ, Hewer A, Phillips DH, Parker P, et al. Tumour necrosis factor-alpha mediates tumour promotion via a PKC alpha- and AP-1-dependent pathway. Oncogene. 2002. 21:4728–4738.
Article24. Pörn MI, Slotte JP. Localization of cholesterol in sphingomyelinase-treated fibroblasts. Biochem J. 1995. 308(Pt 1):269–274.
Article25. Shin MH, Rhie GE, Kim YK, Park CH, Cho KH, Kim KH, et al. H2O2 accumulation by catalase reduction changes MAP kinase signaling in aged human skin in vivo. J Invest Dermatol. 2005. 125:221–229.
Article26. Furuchi T, Anderson RG. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK). J Biol Chem. 1998. 273:21099–21104.
Article27. Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003. 44:655–667.
Article28. Simons K, Ikonen E. How cells handle cholesterol. Science. 2000. 290:1721–1726.
Article29. Wang PY, Liu P, Weng J, Sontag E, Anderson RG. A cholesterol-regulated PP2A/HePTP complex with dual specificity ERK1/2 phosphatase activity. EMBO J. 2003. 22:2658–2667.
Article30. Wang PY, Weng J, Anderson RG. OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science. 2005. 307:1472–1476.
Article31. Kim S, Lee Y, Lee DH, Kim Y, Cho KH, Chung JH. Basal and UV-induced MMP-1 expression are inhibited by p53 in human dermal fibroblasts. Exp Dermatol. 2008. 17:939–945.
Article32. Kim S, Kim Y, Lee Y, Chung JH. Ceramide accelerates ultraviolet-induced MMP-1 expression through JAK1/STAT-1 pathway in cultured human dermal fibroblasts. J Lipid Res. 2008. 49:2571–2581.
Article33. Ravanti L, Häkkinen L, Larjava H, Saarialho-Kere U, Foschi M, Han J, et al. Transforming growth factor-beta induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase. J Biol Chem. 1999. 274:37292–37300.
Article34. Laine P, Reunanen N, Ravanti L, Foschi M, Santra M, Iozzo RV, et al. Activation of extracellular signal-regulated protein kinase1,2 results in down-regulation of decorin expression in fibroblasts. Biochem J. 2000. 349:19–25.
Article35. Park CH, Lee MJ, Ahn J, Kim S, Kim HH, Kim KH, et al. Heat shock-induced matrix metalloproteinase (MMP)-1 and MMP-3 are mediated through ERK and JNK activation and via an autocrine interleukin-6 loop. J Invest Dermatol. 2004. 123:1012–1019.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of tissue inhibitor of matrix metalloproteinase-2 by cholesterol depletion leads to the conversion of proMMP-2 into active MMP-2 in human dermal fibroblasts
- Effects of Hepatocyte Growth Factor on Collagen Synthesis and Matrix Metalloproteinase Production in Keloids
- p-Coumaric Acid Attenuates UVB-Induced Release of Stratifin from Keratinocytes and Indirectly Regulates Matrix Metalloproteinase 1 Release from Fibroblasts
- Ectopic Expression of Caveolin-1 Induces COX-2 Expression in Rabbit Articular Chondrocytes via MAP Kinase Pathway
- Ultraviolet B Downregulated Aquaporin 1 Expression via the MEK/ERK pathway in the Dermal Fibroblasts