Ann Dermatol.
2010 Aug;22(3):290-299. 10.5021/ad.2010.22.3.290.
Epidermal Hyperplasia and Elevated HB-EGF are More Prominent in Retinoid Dermatitis Compared with Irritant Contact Dermatitis Induced by Benzalkonium Chloride
- Affiliations
-
- 1Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea. kimsc@yuhs.ac
- KMID: 2265362
- DOI: http://doi.org/10.5021/ad.2010.22.3.290
Abstract
- BACKGROUND
'Retinoid dermatitis' is a retinoid-induced irritant contact dermatitis (ICD). The mechanism of retinoid dermatitis may be different from that of other ICDs. However, it remains uncertain how topical retinoid induce ICD.
OBJECTIVE
We compared several aspects of contact dermatitis induced by topical retinol and benzalkonium chloride (BKC) on hairless mice skin.
METHODS
2% retinol or 2.5% BKC was applied to hairless mice and transepidermal water loss (TEWL), ear thickness, histologic and immunohistochemical findings were compared. We also compared mRNA expression of inflammatory cytokines, epidermal differential markers, cyclooxygenases (COXs) and heparin binding epidermal growth factor like growth factor (HB-EGF).
RESULTS
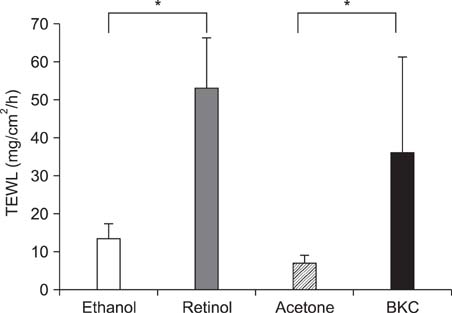
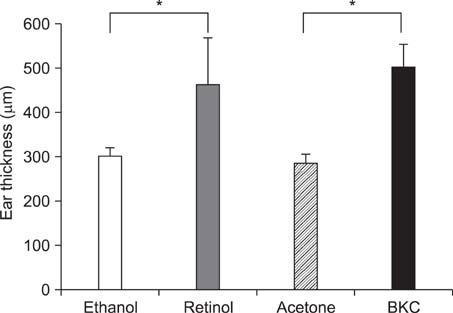
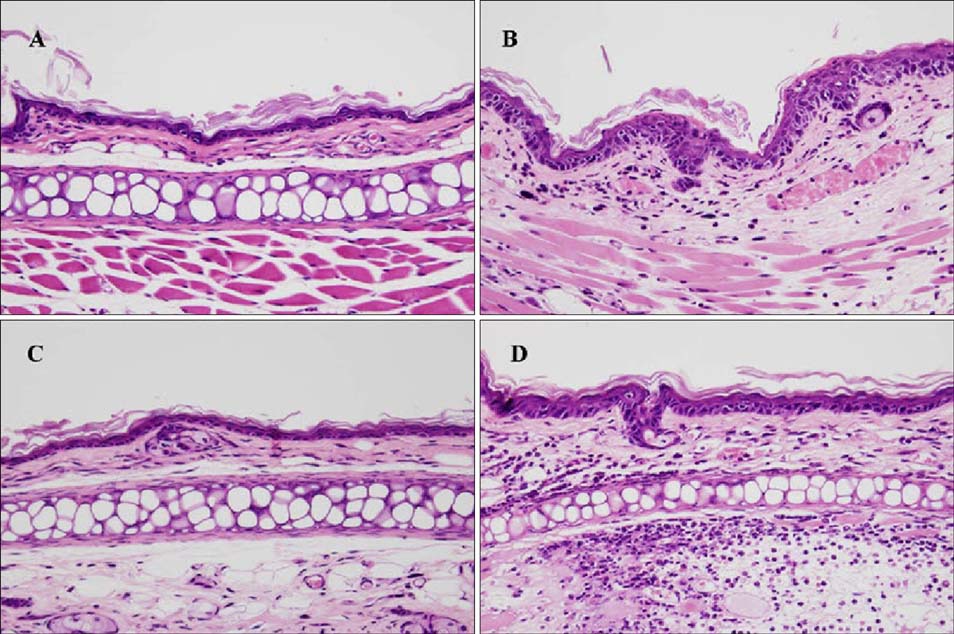
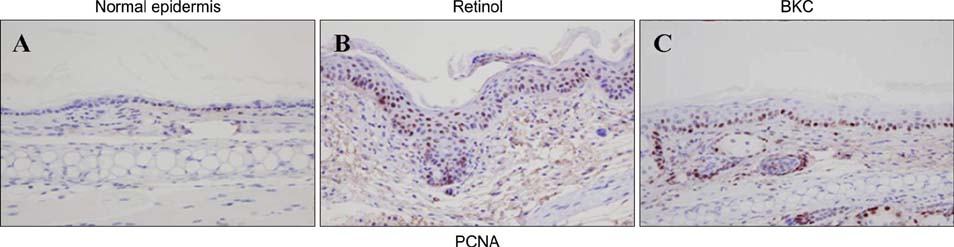
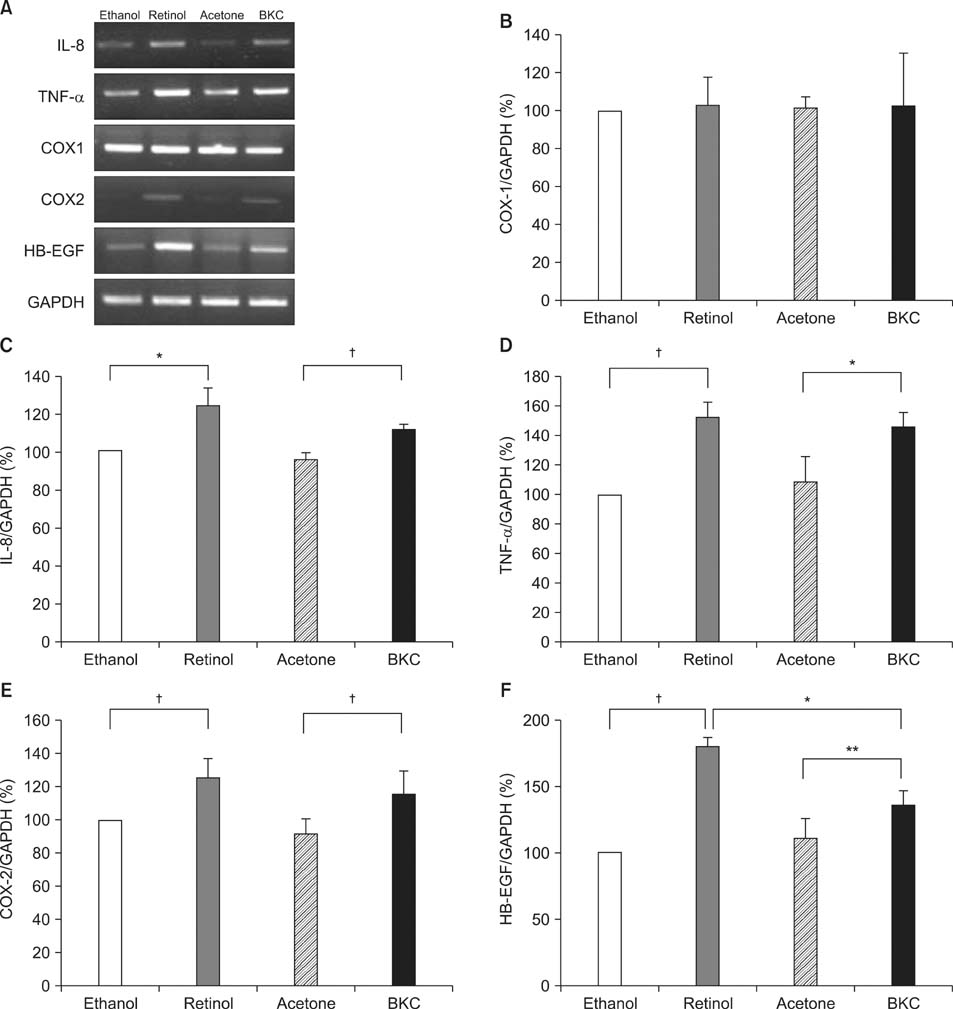
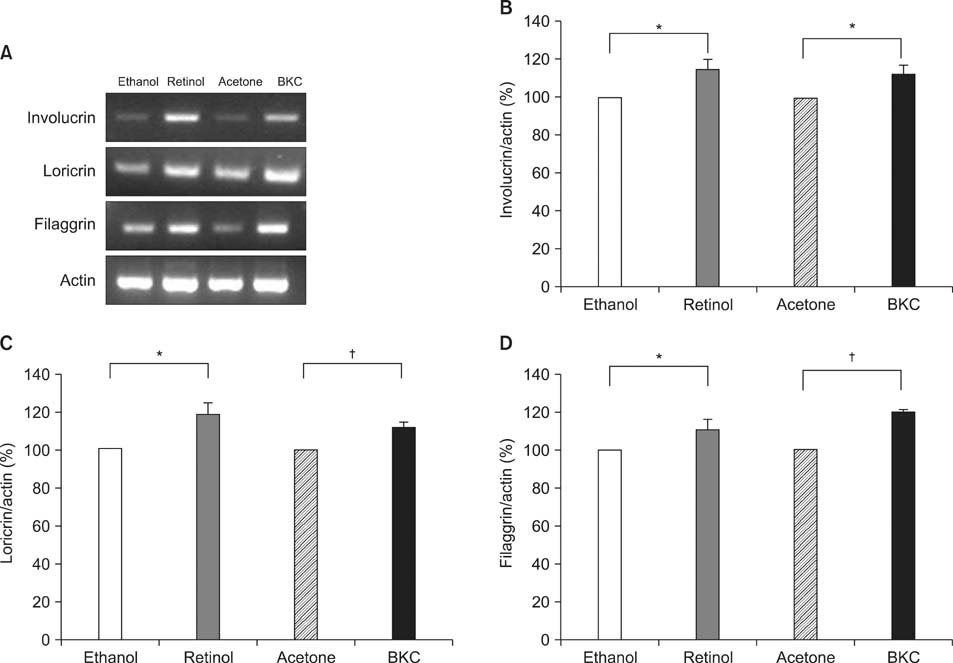
Topical application of 2% retinol and 2.5% BKC increased TEWL and ear thickness in similar intensity. Epidermal hyperplasia was more prominent in retinol treated skin. Proliferating cell nuclear antigen, involucrin and loricrin expression were higher in retinol-treated skin than in BKC-treated skin. Filaggrin, however, was more expressed in BKC-treated skin. The mRNA expression of IL-8, TNF-alpha, COX-2, involucrin, loricrin and filaggrin were increased in both retinol- and BKC-treated skin in similar intensity. HB-EGF was more significantly increased in retinol-treated skin.
CONCLUSION
Elevated HB-EGF and epidermal hyperplasia are more prominent features of retinoid dermatitis than in BKC-induced ICD.
MeSH Terms
-
Animals
Benzalkonium Compounds
Cytokines
Dermatitis
Dermatitis, Contact
Ear
Epidermal Growth Factor
Heparin
Hyperplasia
Intercellular Signaling Peptides and Proteins
Interleukin-8
Intermediate Filament Proteins
Membrane Proteins
Mice
Mice, Hairless
Proliferating Cell Nuclear Antigen
Prostaglandin-Endoperoxide Synthases
Protein Precursors
RNA, Messenger
Skin
Tumor Necrosis Factor-alpha
Vitamin A
Water Loss, Insensible
Benzalkonium Compounds
Cytokines
Epidermal Growth Factor
Heparin
Intercellular Signaling Peptides and Proteins
Interleukin-8
Intermediate Filament Proteins
Membrane Proteins
Proliferating Cell Nuclear Antigen
Prostaglandin-Endoperoxide Synthases
Protein Precursors
RNA, Messenger
Tumor Necrosis Factor-alpha
Vitamin A
Water Loss, Insensible
Figure
Reference
-
1. Berardesca E, Distante F. Mechanisms of skin irritations. Curr Probl Dermatol. 1995. 23:1–8.2. Corsini E, Galli CL. Cytokines and irritant contact dermatitis. Toxicol Lett. 1998. 102-103:277–282.
Article3. Ale SI, Laugier JP, Maibach HI. Differential irritant skin responses to tandem application of topical retinoic acid and sodium lauryl sulphate: II. Effect of time between first and second exposure. Br J Dermatol. 1997. 137:226–233.
Article4. Kligman LH, Sapadin AN, Schwartz E. Peeling agents and irritants, unlike tretinoin, do not stimulate collagen synthesis in the photoaged hairless mouse. Arch Dermatol Res. 1996. 288:615–620.
Article5. Marks R, Hill S, Barton SP. The effects of an abrasive agent on normal skin and on photoaged skin in comparison with topical tretinoin. Br J Dermatol. 1990. 123:457–466.
Article6. Chen S, Ostrowski J, Whiting G, Roalsvig T, Hammer L, Currier SJ, et al. Retinoic acid receptor gamma mediates topical retinoid efficacy and irritation in animal models. J Invest Dermatol. 1995. 104:779–783.
Article7. Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996. 10:1002–1013.
Article8. Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996. 271:33157–33160.
Article9. Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996. 1299:125–140.
Article10. Stoll SW, Elder JT. Retinoid regulation of heparin-binding EGF-like growth factor gene expression in human keratinocytes and skin. Exp Dermatol. 1998. 7:391–397.
Article11. Yoshimura K, Uchida G, Okazaki M, Kitano Y, Harii K. Differential expression of heparin-binding EGF-like growth factor (HB-EGF) mRNA in normal human keratinocytes induced by a variety of natural and synthetic retinoids. Exp Dermatol. 2003. 12:Suppl 2. 28–34.
Article12. Chapellier B, Mark M, Messaddeq N, Calleja C, Warot X, Brocard J, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002. 21:3402–3413.
Article13. Xiao JH, Feng X, Di W, Peng ZH, Li LA, Chambon P, et al. Identification of heparin-binding EGF-like growth factor as a target in intercellular regulation of epidermal basal cell growth by suprabasal retinoic acid receptors. EMBO J. 1999. 18:1539–1548.
Article14. Rittie L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006. 126:732–739.
Article15. Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci U S A. 1992. 89:1398–1402.
Article16. Corsini E, Terzoli A, Bruccoleri A, Marinovich M, Galli CL. Induction of tumor necrosis factor-alpha in vivo by a skin irritant, tributyltin, through activation of transcription factors: its pharmacological modulation by anti-inflammatory drugs. J Invest Dermatol. 1997. 108:892–896.
Article17. Coquette A, Berna N, Vandenbosch A, Rosdy M, De Wever B, Poumay Y. Analysis of interleukin-1alpha (IL-1alpha) and interleukin-8 (IL-8) expression and release in in vitro reconstructed human epidermis for the prediction of in vivo skin irritation and/or sensitization. Toxicol In Vitro. 2003. 17:311–321.
Article18. Kim BH, Lee YS, Kang KS. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol Lett. 2003. 146:65–73.
Article19. Dai X, Yamasaki K, Shirakata Y, Sayama K, Hashimoto K. All-trans-retinoic acid induces interleukin-8 via the nuclear factor-kappaB and p38 mitogen-activated protein kinase pathways in normal human keratinocytes. J Invest Dermatol. 2004. 123:1078–1085.
Article20. Harant H, de Martin R, Andrew PJ, Foglar E, Dittrich C, Lindley IJ. Synergistic activation of interleukin-8 gene transcription by all-trans-retinoic acid and tumor necrosis factor-alpha involves the transcription factor NF-kappaB. J Biol Chem. 1996. 271:26954–26961.
Article21. Xiao R, Kanekura T, Yoshida N, Higashi Y, Yan KL, Fukushige T, et al. 9-Cis-retinoic acid exhibits antifibrotic activity via the induction of cyclooxygenase-2 expression and prostaglandin E2 production in scleroderma fibroblasts. Clin Exp Dermatol. 2008. 33:484–490.
Article22. Kanekura T, Higashi Y, Kanzaki T. Inhibitory effects of 9-cis-retinoic acid and pyrrolidinedithiocarbamate on cyclooxygenase (COX)-2 expression and cell growth in human skin squamous carcinoma cells. Cancer Lett. 2000. 161:177–183.
Article23. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw Hill;2108.24. Fisher LB, Maibach HI. Effect of some irritants on human epidermal mitosis. Contact Dermatitis. 1975. 1:273–276.
Article25. Varani J, Perone P, Spahlinger DM, Singer LM, Diegel KL, Bobrowski WF, et al. Human skin in organ culture and human skin cells (keratinocytes and fibroblasts) in monolayer culture for assessment of chemically induced skin damage. Toxicol Pathol. 2007. 35:693–701.
Article26. Mathay C, Giltaire S, Minner F, Bera E, Herin M, Poumay Y. Heparin-binding EGF-like growth factor is induced by disruption of lipid rafts and oxidative stress in keratinocytes and participates in the epidermal response to cutaneous wounds. J Invest Dermatol. 2008. 128:717–727.
Article27. Floyd EE, Jetten AM. Regulation of type I (epidermal) transglutaminase mRNA levels during squamous differentiation: down regulation by retinoids. Mol Cell Biol. 1989. 9:4846–4851.
Article28. Asselineau D, Dale BA, Bernard BA. Filaggrin production by cultured human epidermal keratinocytes and its regulation by retinoic acid. Differentiation. 1990. 45:221–229.
Article29. Magnaldo T, Bernerd F, Asselineau D, Darmon M. Expression of loricrin is negatively controlled by retinoic acid in human epidermis reconstructed in vitro. Differentiation. 1992. 49:39–46.
Article30. Hohl D, de Viragh PA, Amiguet-Barras F, Gibbs S, Backendorf C, Huber M. The small proline-rich proteins constitute a multigene family of differentially regulated cornified cell envelope precursor proteins. J Invest Dermatol. 1995. 104:902–909.
Article31. Rosenthal DS, Griffiths CE, Yuspa SH, Roop DR, Voorhees JJ. Acute or chronic topical retinoic acid treatment of human skin in vivo alters the expression of epidermal transglutaminase, loricrin, involucrin, filaggrin, and keratins 6 and 13 but not keratins 1, 10, and 14. J Invest Dermatol. 1992. 98:343–350.
Article32. Griffiths CE, Rosenthal DS, Reddy AP, Elder JT, Astrom A, Leach K, et al. Short-term retinoic acid treatment increases in vivo, but decreases in vitro, epidermal transglutaminase-K enzyme activity and immunoreactivity. J Invest Dermatol. 1992. 99:283–288.
Article33. Le M, Schalkwijk J, Siegenthaler G, van de Kerkhof PC, Veerkamp JH, van der Valk PG. Changes in keratinocyte differentiation following mild irritation by sodium dodecyl sulphate. Arch Dermatol Res. 1996. 288:684–690.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Irritant Contact Dermatitis Induced by Benzalkonium Chloride (Zephanon®)
- A Case of Irritant Contact Dermafitis Due to Ranuculus japonicus
- A Case of Irritant Contact Dermatitis due to Fiberglass in a Construction Worker
- Allergic contact dermatitis to benzalkonium chloride in eyedrops
- Two Cases of Irritant Contact Dermatitis due to Garlic