Interactions Between Innate Immunity Genes and Early-Life Risk Factors in Allergic Rhinitis
- Affiliations
-
- 1Department of Pediatrics, Korean Cancer Center Hospital, Seoul, Korea.
- 2Department of Pediatrics, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 3Department of Pediatrics, CHA University School of Medicine, Seongnam, Korea.
- 4Childhood Asthma Atopy Center, Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sjhong@amc.seoul.kr
- 5Research Center for Standardization of Allergic Diseases, University of Ulsan College of Medicine, Seoul, Korea.
- 6Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
- 7Department of Pediatrics, Presbyterian Medical Center, Jeonju, Korea.
- 8Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea.
- 9Department of Pediatrics, Inje University Haeundae Paik Hospital, Busan, Korea.
- 10Department of Pediatrics, Inje University Sanggye Paik Hospital, Seoul, Korea.
- 11Allergy TF, Department of Immunology and Pathology, Korea National Institute of Health, Osong, Korea.
- 12Department of Pediatrics, Hallym Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea. imipenem@hanmail.net
- KMID: 2260461
- DOI: http://doi.org/10.4168/aair.2015.7.3.241
Abstract
- PURPOSE
Allergic rhinitis (AR) is a common chronic disease. Many factors could affect the development of AR. We investigated early-life factors, such as delivery mode, feeding method, and use of antibiotics during infancy, which could affect the development of AR. In addition, how interactions between these factors and innate gene polymorphisms influence the development of AR was investigated.
METHODS
A cross-sectional study of 1,828 children aged 9-12 years was conducted. Three early-life factors and AR were assessed by a questionnaire. Skin prick tests were done. Polymorphisms of TLR4 (rs1927911) and CD14 (rs2569190) were genotyped.
RESULTS
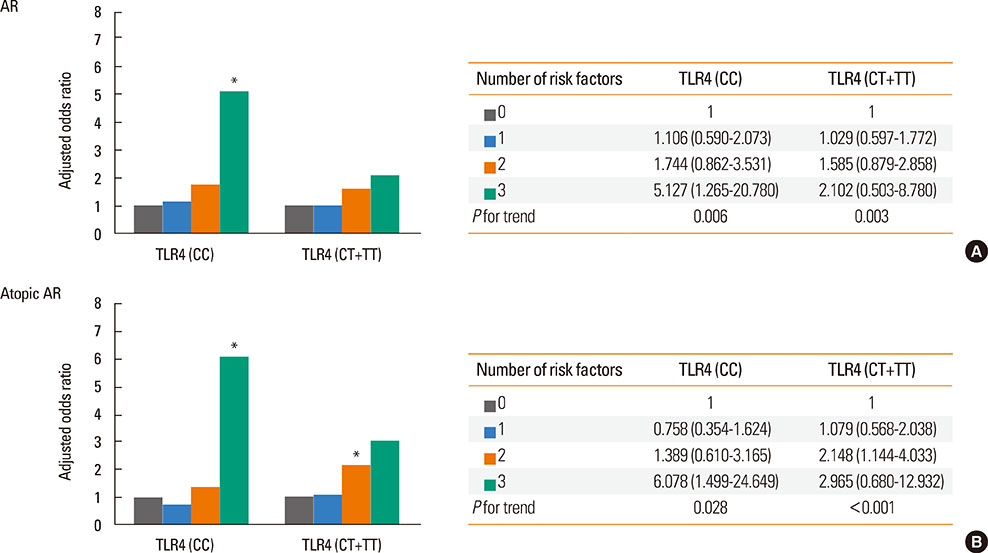
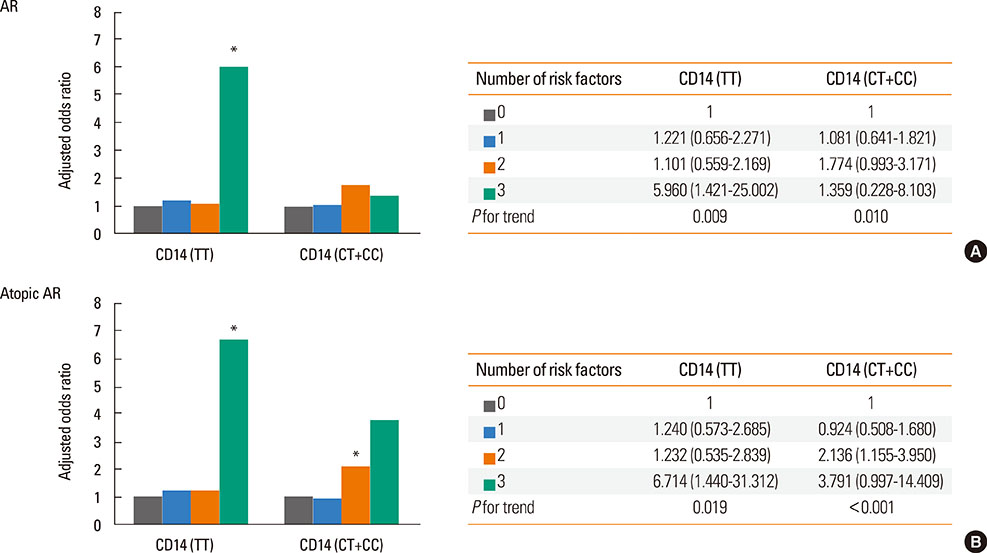
Use of antibiotics during infancy increased the risk of AR (aOR [95% CI] 1.511 [1.222-2.037]) and atopic AR (aOR [95% CI], 1.565 [1.078-2.272]). There were synergistic interactions between caesarean delivery, formula feeding, and use of antibiotics in the rate of atopic AR (aOR [95% CI], 3.038 [1.256-7.347]). Additional analyses revealed that the risk for the development of AR or atopic AR subjects with the TLR4 CC genotype were highest when all the 3 early-life factors were present (aOR [95% CI], 5.127 [1.265-20.780] for AR; 6.078 [1.499-24.649] for atopic AR). In addition, the risk for the development of AR or atopic AR in subjects with the CD14 TT genotype were highest when all the 3 early-life factors were present (aOR [95% CI], 5.960 [1.421-15.002] for AR; 6.714 [1.440-31.312] for atopic AR).
CONCLUSIONS
Delivery mode, feeding method, and use of antibiotics during infancy appeared to have synergistic interactions in the development of AR. Gene-environment interactions between polymorphism of innate genes and early- life risk factors might affect the development of AR.
Keyword
MeSH Terms
Figure
Cited by 5 articles
-
The prevalence and risk factors of allergic rhinitis from a nationwide study of Korean elementary, middle, and high school students
Yeongho Kim, Ju-Hee Seo, Ji-Won Kwon, Eun Lee, Song-I Yang, Hyun-Ju Cho, Mina Ha, Eunae Burm, Kee-Jae Lee, Hwan-Cheol Kim, Sinye Lim, Hee-Tae Kang, Mia Son, Soo-Young Kim, Hae-Kwan Cheong, Yu-Mi Kim, Gyung-Jae Oh, Joon Sakong, Chul-Gab Lee, Sue Jin Kim, Yong-Wook Beak, Soo-Jong Hong
Allergy Asthma Respir Dis. 2015;3(4):272-280. doi: 10.4168/aard.2015.3.4.272.The change in food allergy prevalence of elementary school children in Seoul since the last 20 years and the risk factor analysis
Yeong-Ho Kim, So-Yeon Lee, Eun Lee, Hyun-Ju Cho, Hyo-Bin Kim, Ji-Won Kwon, Song-I Yang, Eun-Jin Kim, Jeom-Kyu Lee, Soo-Jong Hong
Allergy Asthma Respir Dis. 2016;4(4):276-283. doi: 10.4168/aard.2016.4.4.276.Research on pediatric allergic rhinitis in Korea
Kyung Suk Lee, Yeong Ho Rha
Allergy Asthma Respir Dis. 2018;6(Suppl 1):S58-S65. doi: 10.4168/aard.2018.6.S1.S58.Overview and challenges of current genetic research on allergic diseases in Korean children
Myunghyun Sohn
Allergy Asthma Respir Dis. 2018;6(Suppl 1):S77-S84. doi: 10.4168/aard.2018.6.S1.S77.Prenatal Maternal Distress and Allergic Diseases in Offspring: Review of Evidence and Possible Pathways
Dong In Suh, Hyoung Yoon Chang, Eun Lee, Song-I Yang, Soo-Jong Hong
Allergy Asthma Immunol Res. 2017;9(3):200-211. doi: 10.4168/aair.2017.9.3.200.
Reference
-
1. Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Korean J Pediatr. 2008; 51:343–350.2. Kim WK, Kwon JW, Seo JH, Kim HY, Yu J, Kim BJ, et al. Interaction between IL13 genotype and environmental factors in the risk for allergic rhinitis in Korean children. J Allergy Clin Immunol. 2012; 130:421–426.e5.3. Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989; 299:1259–1260.4. Lee SY, Kwon JW, Seo JH, Song YH, Kim BJ, Yu J, et al. Prevalence of atopy and allergic diseases in Korean children: associations with a farming environment and rural lifestyle. Int Arch Allergy Immunol. 2012; 158:168–174.5. Orrhage K, Nord CE. Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr Suppl. 1999; 88:47–57.6. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010; 107:11971–11975.7. Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006; 118:511–521.8. Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. 2010; 51:77–84.9. Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol. 2011; 9:233–243.10. Murgas Torrazza R, Neu J. The developing intestinal microbiome and its relationship to health and disease in the neonate. J Perinatol. 2011; 31:Suppl 1. S29–S34.11. Koppelman GH, Reijmerink NE, Colin Stine O, Howard TD, Whittaker PA, Meyers DA, et al. Association of a promoter polymorphism of the CD14 gene and atopy. Am J Respir Crit Care Med. 2001; 163:965–969.12. Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A Polymorphism* in the 5' flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol. 1999; 20:976–983.13. Tesse R, Pandey RC, Kabesch M. Genetic variations in toll-like receptor pathway genes influence asthma and atopy. Allergy. 2011; 66:307–316.14. Choi WA, Kang MJ, Kim YJ, Seo JH, Kim HY, Kwon JW, et al. Gene-gene interactions between candidate gene polymorphisms are associated with total IgE levels in Korean children with asthma. J Asthma. 2012; 49:243–252.15. Hong SJ, Kim HB, Kang MJ, Lee SY, Kim JH, Kim BS, et al. TNF-alpha (-308 G/A) and CD14 (-159T/C) polymorphisms in the bronchial responsiveness of Korean children with asthma. J Allergy Clin Immunol. 2007; 119:398–404.16. Kim HY, Jung YH, Hong K, Jang GC, Seo JH, Kwon JW, et al. Gene-environment interaction between Toll-like receptor 4 and mold exposure in the development of atopic dermatitis in preschool children. Allergy Asthma Respir Dis. 2013; 1:129–137.17. Jones G. Susceptibility to asthma and eczema from mucosal and epidermal expression of distinctive genes. Curr Allergy Asthma Rep. 2007; 7:11–17.18. Hong SJ, Kim SW, Oh JW, Rah YH, Ahn YM, Kim KE, et al. The validity of the ISAAC written questionnaire and the ISAAC video questionnaire (AVQ 3.0) for predicting asthma associated with bronchial hyperreactivity in a group of 13-14 year old Korean schoolchildren. J Korean Med Sci. 2003; 18:48–52.19. Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003; 361:512–519.20. Adlerberth I, Strachan DP, Matricardi PM, Ahrné S, Orfei L, Aberg N, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007; 120:343–350.21. Savilahti E, Tainio VM, Salmenperä L, Siimes MA, Perheentupa J. Prolonged exclusive breast feeding and heredity as determinants in infantile atopy. Arch Dis Child. 1987; 62:269–273.22. Savilahti E. Interaction of early infant feeding, heredity and other environmental factors as determinants in the development of allergy and sensitization. Nestle Nutr Workshop Ser Pediatr Program. 2008; 62:157–168.23. Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012; 9:565–576.24. Wjst M, Hoelscher B, Frye C, Wichmann HE, Dold S, Heinrich J. Early antibiotic treatment and later asthma. Eur J Med Res. 2001; 6:263–271.25. McKeever TM, Lewis SA, Smith C, Collins J, Heatlie H, Frischer M, et al. Early exposure to infections and antibiotics and the incidence of allergic disease: a birth cohort study with the West Midlands General Practice Research Database. J Allergy Clin Immunol. 2002; 109:43–50.26. Shreiner A, Huffnagle GB, Noverr MC. The "Microflora Hypothesis" of allergic disease. Adv Exp Med Biol. 2008; 635:113–134.27. Verhulst SL, Vael C, Beunckens C, Nelen V, Goossens H, Desager K. A longitudinal analysis on the association between antibiotic use, intestinal microflora, and wheezing during the first year of life. J Asthma. 2008; 45:828–832.28. Kozyrskyj AL, Ernst P, Becker AB. Increased risk of childhood asthma from antibiotic use in early life. Chest. 2007; 131:1753–1759.29. Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012; 13:440–447.30. Shen CM, Lin SC, Niu DM, Kou YR. Labour increases the surface expression of two Toll-like receptors in the cord blood monocytes of healthy term newborns. Acta Paediatr. 2009; 98:959–962.31. Belderbos ME, Houben ML, van Bleek GM, Schuijff L, van Uden NO, Bloemen-Carlier EM, et al. Breastfeeding modulates neonatal innate immune responses: a prospective birth cohort study. Pediatr Allergy Immunol. 2012; 23:65–74.32. Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008; 455:804–807.33. Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011; 332:974–977.34. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004; 118:229–241.35. Shirai Y, Hashimoto M, Kato R, Kawamura YI, Kirikae T, Yano H, et al. Lipopolysaccharide induces CD25-positive, IL-10-producing lymphocytes without secretion of proinflammatory cytokines in the human colon: low MD-2 mRNA expression in colonic macrophages. J Clin Immunol. 2004; 24:42–52.36. McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. J Allergy Clin Immunol. 2011; 127:1097–1107.37. Wells JM, Loonen LM, Karczewski JM. The role of innate signaling in the homeostasis of tolerance and immunity in the intestine. Int J Med Microbiol. 2010; 300:41–48.38. Penders J, Thijs C, Mommers M, Stobberingh EE, Dompeling E, Reijmerink NE, et al. Host-microbial interactions in childhood atopy: toll-like receptor 4 (TLR4), CD14, and fecal Escherichia coli. J Allergy Clin Immunol. 2010; 125:231–236.e1-5.39. Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000; 25:187–191.40. Kerkhof M, Postma DS, Brunekreef B, Reijmerink NE, Wijga AH, de Jongste JC, et al. Toll-like receptor 2 and 4 genes influence susceptibility to adverse effects of traffic-related air pollution on childhood asthma. Thorax. 2010; 65:690–697.41. Sly PD, Holt PG. Role of innate immunity in the development of allergy and asthma. Curr Opin Allergy Clin Immunol. 2011; 11:127–131.42. Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrländer C, et al. Opposite effects of CD 14/-260 on serum IgE levels in children raised in different environments. J Allergy Clin Immunol. 2005; 116:601–607.43. Simpson A, John SL, Jury F, Niven R, Woodcock A, Ollier WE, et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006; 174:386–392.44. Corver K, Kerkhof M, Brussee JE, Brunekreef B, van Strien RT, Vos AP, et al. House dust mite allergen reduction and allergy at 4 yr: follow up of the PIAMA-study. Pediatr Allergy Immunol. 2006; 17:329–336.45. Woodcock A, Lowe LA, Murray CS, Simpson BM, Pipis SD, Kissen P, et al. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med. 2004; 170:433–439.46. Chan-Yeung M, Ferguson A, Watson W, Dimich-Ward H, Rousseau R, Lilley M, et al. The Canadian Childhood Asthma Primary Prevention Study: outcomes at 7 years of age. J Allergy Clin Immunol. 2005; 116:49–55.