Allergy Asthma Immunol Res.
2010 Jan;2(1):1-13. 10.4168/aair.2010.2.1.1.
Current guidelines for the management of asthma in young children
- Affiliations
-
- 1Allergy Diagnostic & Clinical Research Unit, University of Cape Town Lung Institute, Cape Town, South Africa. Paul.Potter@uct.ac.za
- KMID: 2260413
- DOI: http://doi.org/10.4168/aair.2010.2.1.1
Abstract
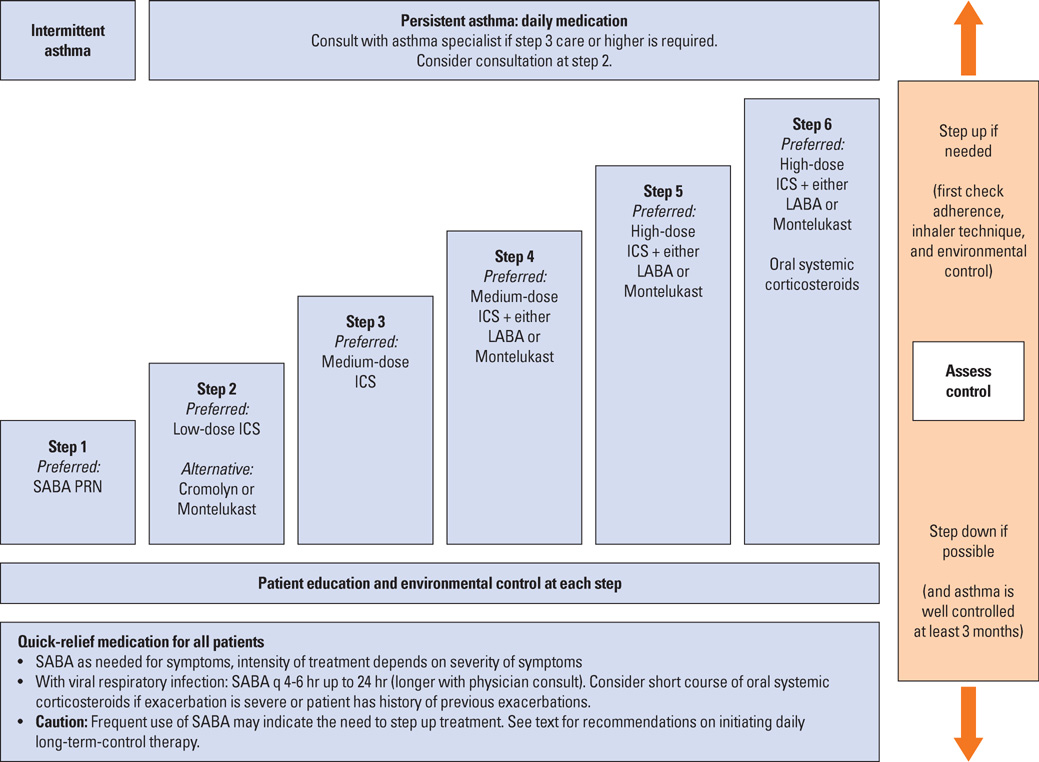
- The diagnosis and management of asthma in young children is difficult, since there are many different wheezy phenotypes with varying underlying aetiologies and outcomes. This review discusses the different approaches to managing young children with wheezy illnesses presented in recently published global guidelines. Four major guidelines published since 2007 are considered. Helpful approaches are presented to assist the clinician to decide whether a clinical diagnosis of asthma can, or should be made in a young child with a recurrent wheezy illness and which treatments would be appropriate, dependent on risk factors, age of presentation, response to initial treatment and safety considerations. Each of the guidelines provide useful information for clinicians assessing young children with recurrent wheezy illnesses. There are differences in classification of the disease and treatment protocols. Although a firm diagnosis of asthma may only be made retrospectively in some cases and there are several effective guidelines to initiating treatment. Consistent review of the need for ongoing treatment with a particular pharmacological modality is essential, since many children with recurrent wheezing in infancy go into spontaneous remission. It is probable that newer biomarkers of airway inflammation will assist the clinician as to when to initiate and when to continue pharmacological treatment in the future.
Keyword
MeSH Terms
Figure
Reference
-
1. Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006. 368:733–743.2. EPR. Expert panel report: guidelines for the diagnosis and management of asthma (EPR 1991). 1991. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program;NIH Publication No. 91-3642.3. EPR-2. Expert panel report 2: guidelines for the diagnosis and management of asthma (EPR-2 1997). 1997. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program;NIH Publication No. 97-4051.4. Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, Stevens R. Systemic reviews and meta-analysis on treatment of asthma: critical evaluation. BMJ. 2000. 320:537–540.5. EPR-Update 2002. Expert panel report: guidelines for the diagnosis and management of asthma. Update on selected topics 2002 (EPR-Update 2002). 2003. 06. Bethesda, MD: U.S. Department of Health and Human Services; National Institutes of Health; National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program;NIH Publication No. 02 5074.6. NIH. National Asthma Education and Prevention Program. Expert Panel Report III: Guidelines for the Diagnosis and Management of Asthma. 2007. Bethesda, MD: National Institutes of Health; National Heart, Lung, and Blood Institute;NIH Publication No. 07-4051.7. Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Gotz M, Helms PJ, Hunt J, Liu A, Papadopoulos N, Platts-Mills T, Pohunek P, Simons FE, Valovirta E, Wahn U, Wildhaber J. European Pediatric Asthma Group. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008. 63:5–34.8. Brand PL, Baraldi E, Bisgaard H, Boner AL, Castro-Rodriguez JA, Custovic A, de Blic J, de Jongste JC, Eber E, Everard ML, Frey U, Gappa M, Garcia-Marcos L, Grigg J, Lenney W, Le Souef P, McKenzie S, Merkus PJ, Midulla F, Paton JY, Piacentini G, Pohunek P, Rossi GA, Seddon P, Silverman M, Sly PD, Stick S, Valiulis A, van Aalderen WM, Wildhaber JH, Wennergren G, Wilson N, Zivkovic Z, Bush A. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J. 2008. 32:1096–1110.9. Global Initiative for Asthma: Global strategy for the diagnosis and management of asthma in children 5 years and younger [Internet]. 2009. Available from: www.ginasthma.org.10. Borish L, Culp JA. Asthma: a syndrome composed of heterogeneous diseases. Ann Allergy Asthma Immunol. 2008. 101:1–8. quiz 8-11, 50.11. Ait-Khaled N, Enarson DA, Bissell K, Billo NE. Access to inhaled corticosteroids is key to improving quality of care for asthma in developing countries. Allergy. 2007. 62:230–236.12. Levin ME. Language as a barrier to care for Xhosa-speaking patients at a South African paediatric teaching hospital. S Afr Med J. 2006. 96:1076–1079.13. Bateman ED. Severity and control of severe asthma. J Allergy Clin Immunol. 2006. 117:519–521.14. Tan E, Cranswick NE, Rayner CR, Chapman CB. Dosing information for paediatric patients: are they really "therapeutic orphans"? Med J Aust. 2003. 179:195–198.15. Krawiec ME, Westcott JY, Chu HW, Balzar S, Trudeau JB, Schwartz LB, Wenzel SE. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001. 163:1338–1343.16. Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF Jr, Strunk RC, Allen DB, Bloomberg GR, Heldt G, Krawiec M, Larsen G, Liu AH, Chinchilli VM, Sorkness CA, Taussig LM, Martinez FD. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006. 354:1985–1997.17. Bisgaard H, Szefler S. Long-acting beta2 agonists and paediatric asthma. Lancet. 2006. 367:286–288.18. Covar RA, Spahn JD, Murphy JR, Szefler SJ. Childhood Asthma Management Program Research Group. Progression of asthma measured by lung function in the childhood asthma management program. Am J Respir Crit Care Med. 2004. 170:234–241.19. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995. 332:133–138.20. Agertoft L, Pedersen S. Effect of long-term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med. 2000. 343:1064–1069.21. Gillman SA, Anolik R, Schenkel E, Newman K. One-year trial on safety and normal linear growth with flunisolide HFA in children with asthma. Clin Pediatr (Phila). 2002. 41:333–340.22. Rodrigo GJ, Castro-Rodriguez JA. Anticholinergics in the treatment of children and adults with acute asthma: a systematic review with meta-analysis. Thorax. 2005. 60:740–746.23. Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, Michele TM, Reiss TF, Nguyen HH, Bratton DL. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics. 2001. 108:E48.24. Straub DA, Minocchieri S, Moeller A, Hamacher J, Wildhaber JH. The effect of montelukast on exhaled nitric oxide and lung function in asthmatic children 2 to 5 years old. Chest. 2005. 127:509–514.25. Simons FE, Villa JR, Lee BW, Teper AM, Lyttle B, Aristizabal G, Laessig W, Schuster A, Perez-Frias J, Sekerel BE, Menten J, Leff JA. Montelukast added to budesonide in children with persistent asthma: a randomized, double-blind, crossover study. J Pediatr. 2001. 138:694–698.26. Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. The Dutch Asthma Study Group. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. Am J Respir Crit Care Med. 1998. 158:213–219.27. Bisgaard H, Szefler S. Long-acting beta2 agonists and paediatric asthma. Lancet. 2006. 367:286–288.28. Olaguibel JM, Alvarez Puebla MJ. Efficacy of sublingual allergen vaccination for respiratory allergy in children. Conclusions from one meta-analysis. J Investig Allergol Clin Immunol. 2005. 15:9–16.29. Di Rienzo V, Marcucci F, Puccinelli P, Parmiani S, Frati F, Sensi L, Canonica GW, Passalacqua G. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective study. Clin Exp Allergy. 2003. 33:206–210.30. Ducharme FM, Davis GM, Noya F, Rich H, Ernst P. The Asthma Quiz for Kidz: a validated tool to appreciate the level of asthma control in children. Can Respir J. 2004. 11:541–546.31. Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Rosenzweig JC, Manjunath R. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007. 119:817–825.32. Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. 2007. 42:723–728.33. Bush A. Coughs and wheezes spread diseases: but what about the environment? Thorax. 2006. 61:367–368.34. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW Jr, Zaza S. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004. 328:1490.35. Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention [Internet]. 2008. cited 2008 Jul 27. Available from: www.ginasthma.org.36. Martinez FD, Godfrey S. Wheezing disorders in the preschool child: Epidemiology, Diagnosis and Treatment. 2003. London: Martin Dunitz.37. Eysink PE, ter Riet G, Aalberse RC, van Aalderen WM, Roos CM, van der Zee JS, Bindels PJ. Accuracy of specific IgE in the prediction of asthma: development of a scoring formula for general practice. Br J Gen Pract. 2005. 55:125–131.38. Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005. 115:1130–1136.39. Wilhaber H, Sennhauser FH, Brand PL. Frey U, Gerritsen J, editors. Asthma in school aged children and adolescents. Respiratory Diseases in Infants and Children (European Respiratory Monograph). 2006. Philadelphia: Old City Publishing.40. Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, Jeffery PK. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007. 176:858–864.41. Gore RB, Custovic A. Is allergen avoidance effective? Clin Exp Allergy. 2002. 32:662–666.42. Stevens CA, Wesseldine LJ, Couriel JM, Dyer AJ, Osman LM, Silverman M. Parental education and guided self-management of asthma and wheezing in the pre-school child: a randomised controlled trial. Thorax. 2002. 57:39–44.43. Kaditis AG, Winnie G, Syrogiannopoulos GA. Anti-inflammatory pharmacotherapy for wheezing in preschool children. Pediatr Pulmonol. 2007. 42:407–420.44. Hofhuis W, van der Wiel EC, Nieuwhof EM, Hop WC, Affourtit MJ, Smit FJ, Vaessen-Verberne AA, Versteegh FG, de Jongste JC, Merkus PJ. Anti-Inflammatory Treatment in Infants with Recurrent Wheeze (AIR) Study Group. Efficacy of fluticasone propionate on lung function and symptoms in wheezy infants. Am J Respir Crit Care Med. 2005. 171:328–333.45. Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R 3rd, Stout J, Malindzak G, Smartt E, Plaut M, Walter M, Vaughn B, Mitchell H. Inner-City Asthma Study Group. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004. 351:1068–1080.46. Salo PM, Arbes SJ Jr, Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006. 118:892–898.47. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000. 162:1403–1406.48. Zemek RL, Bhogal SK, Ducharme FM. Systematic review of randomized controlled trials examining written action plans in children: what is the plan? Arch Pediatr Adolesc Med. 2008. 162:157–163.49. Baiardini I, Braido F, Bonini M, Compalati E, Canonica GW. Why do doctors and patients not follow guidelines? Curr Opin Allergy Clin Immunol. 2009. 9:228–233.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evolution of Asthma Concept and Effect of Current Asthma Management Guidelines
- Diagnosis and management of asthma in infants and preschoolers
- Knowledge and Practice in Self-Management on Asthma of School-Aged Children with Asthma
- Asthma insights and reality in Korea
- Survey on the Current Status of Asthma Maintenance Therapy and the Impact of Asthma on Children and Family Life