Allergy Asthma Immunol Res.
2010 Oct;2(4):235-246. 10.4168/aair.2010.2.4.235.
A Role of Staphyococcus aureus, Interleukin-18, Nerve Growth Factor and Semaphorin 3A, an Axon Guidance Molecule, in Pathogenesis and Treatment of Atopic Dermatitis
- Affiliations
-
- 1Department of Environmental Immuno-Dermatology, Yokohama City University Graduate School of Medicine, Yokohama, Japan. ik4512@med.yokohama-cu.ac.jp
- 2Life Science Research Center, Shiseido Co. Ltd., Yokohama, Japan.
- KMID: 2260384
- DOI: http://doi.org/10.4168/aair.2010.2.4.235
Abstract
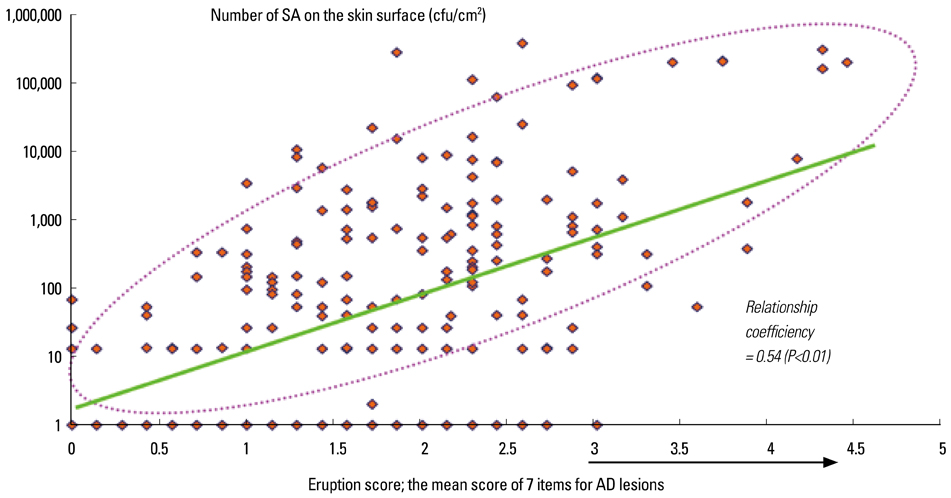
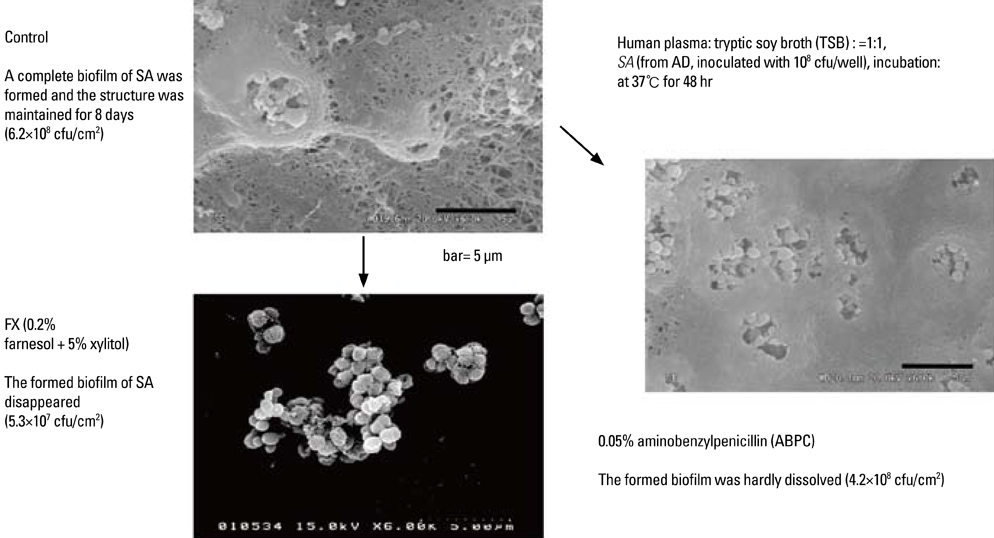
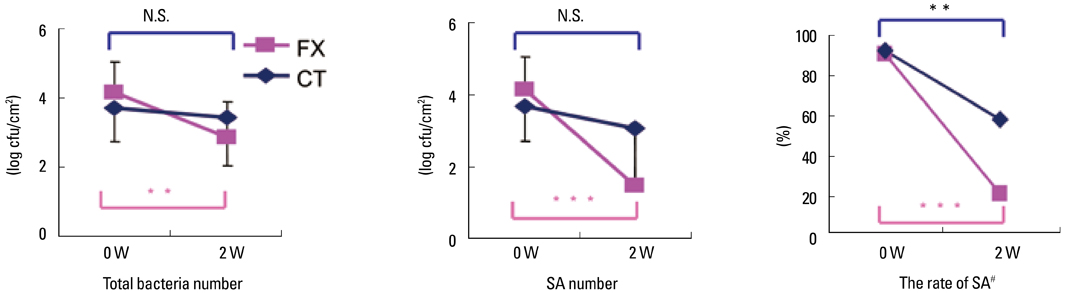
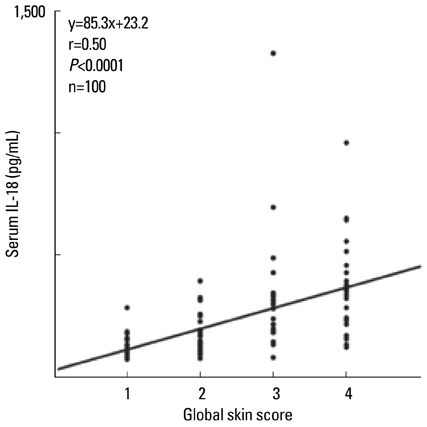
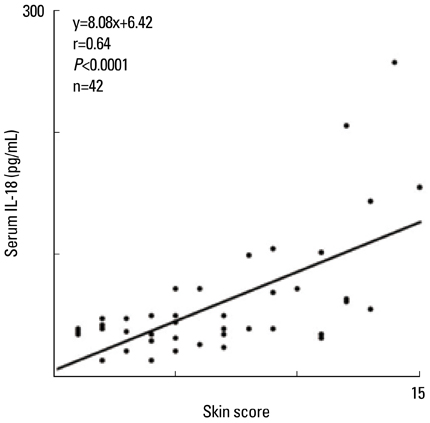
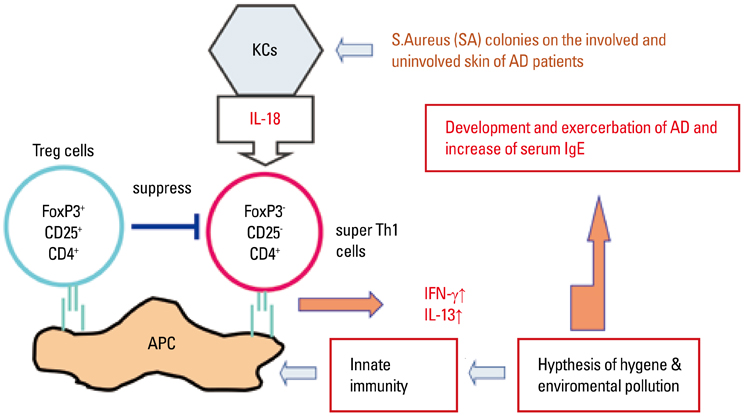
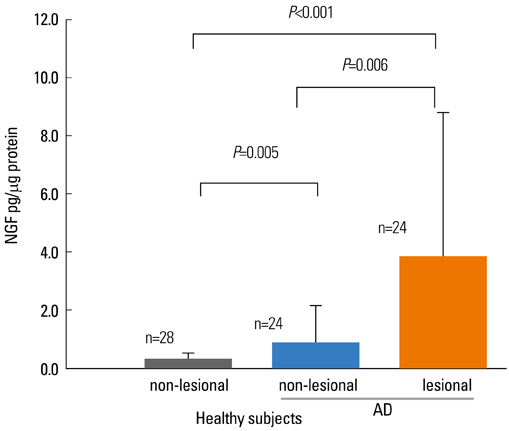
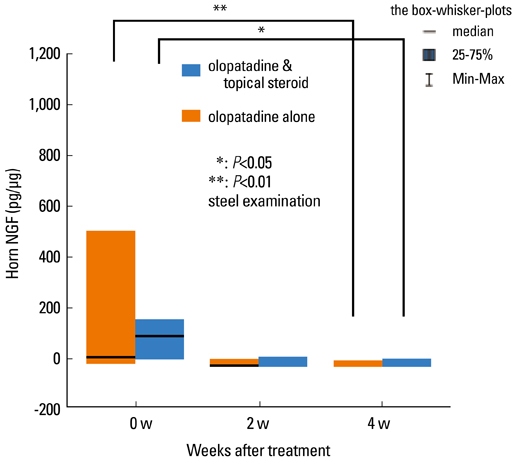
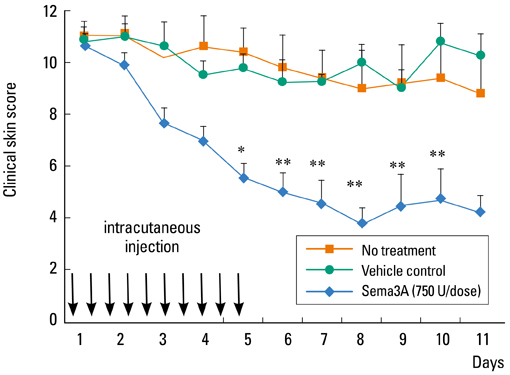
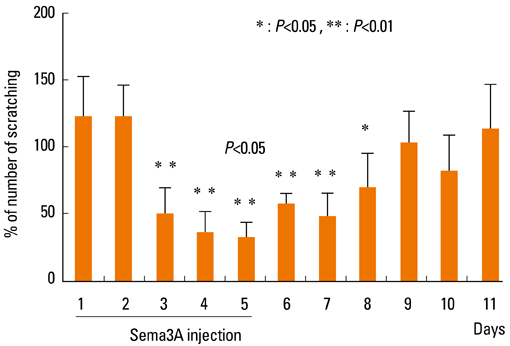
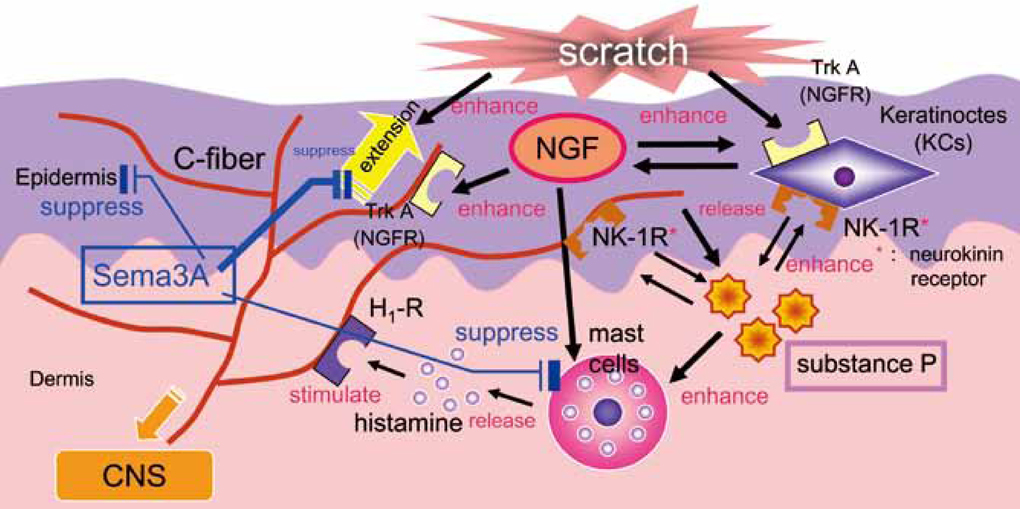
- Staphylococcus aureus (SA) is usually present not only in the skin lesions of atopic dermatitis (AD) but also in the atopic dry skin. SA discharges various toxins and enzymes that injure the skin, results in activation of epidermal keratinocytes, which produce and release IL-18. IL-18 that induces the super Th1 cells secreting IFN-gamma and IL-13 is supposed to be involved in development of AD and its pathogenesis. Indeed, the number of SA colonies on the skin surface and the serum IL-18 levels in patients with AD significantly correlated with the skin scores of AD lesions. Also, there is strong positive correlation between the skin scores and serum IL-18 levels in DS-Nh mice (P<0.0001, r=0.64), which develop considerable AD-like legions when they are housed under conventional conditions, but develop skin legions with less severity and less frequency under specific pathogens free (SPF) conditions. Therefore, they are well-known as model mice of AD, in which SA is presumed to be critical factor for the development of AD lesions. Also, theses DS-Nh mice pretreated with Cy developed more remarkable AD-like lesions in comparison with non-treated ones. The levels of INF-r and IL-13 in the supernatants of the lymph node cell cultures stimulated with staphylococcal enterotoxin B (SEB) or ConA were increased in the Cy-treated mice, although the serum levels of total IgE were not. In this experiment, we revealed that Cy-treated mice, to which CD25 +CD4 + reguratory T cells taken from non-treated ones had been transferred, developed the AD-like legions with less severity and less number of SA colonies on the skin surface. Therefore, it is presumed that CD25 +CD4 + reguratory T cells might be involved in the suppression of super Th1 cells which are induced by IL-18 and are involved in the development of AD-like lesions rather than IgE production. The efficient induction of CD25 +CD4 + reguratory T cells is expected for the new type of treatment of AD. We also found that farnesol (F) and xylitol (X) synergistically inhibited biofilm formation by SA, and indeed the ratio of SA in total bacteria at sites to which the FX cream containing F and X had been applied was significantly decreased 1 week later, accompanied with improvement of AD, when compared with that before application and at placebo sites. Therefore, the FX cream is a useful skin-care agent for atopic dry skin colonized by SA. The nerve growth factor (NGF) in the horny layer (the horn NGF) of skin lesions on the cubital fossa was collected by tape stripping and measured using ELISA in AD patients before and after 2 and 4 weeks treatments. Simultaneously, the itch and eruptions on the whole body and on the lesions, in which the horn NGF was measured, were recorded, and also the peripheral blood eosinophil count, serum LDH level and serum total IgE level were examined. The level of NGF was significantly higher in AD patients than in healthy controls, correlated with the severity of itch, erythema, scale/xerosis, the eosinophil count and LDH level, and also significantly decreased after treatments with olopatadine and/or steroid ointment for 2 and 4 weeks. Therefore, the measurement of the NGF by this harmless method seems to be useful to assess the severity of AD and the therapeutic effects on AD. In AD patients, C-fiber in the epidermis increase and sprout, inducing hypersensitivity, which is considered to aggravate the disease. Semaphorin 3A (Sema3A), an axon guidance molecule, is a potent inhibitor of neurite outgrowth of sensory neurons. We administered recombinant Sema3A intracutaneously into the skin lesions of NC/Nga mice, an animal model of AD, and investigated the effect of Sema3A on the skin lesions and their itch. Sema3A dose-dependently improved skin lesions and attenuated the scratching behavior in NC/Nga mice. Histological examinations revealed a decrease in the epidermal thickness, the density of invasive nerve fibers in the epidermis, inflammatory infiltrate including mast cells and CD4 +T cells, and the production of IL-4 in the Sema3A-treated lesions. Because the interruption of the itch-scratch cycle likely contributes to the improvement of the AD-like lesions, Sema3A is expected to become a promising treatment of patients with refractory AD.
MeSH Terms
-
Animals
Axons
Bacteria
Biofilms
Cell Culture Techniques
Colon
Dermatitis, Atopic
Dibenzoxepins
Enterotoxins
Enzyme-Linked Immunosorbent Assay
Eosinophils
Epidermis
Erythema
Farnesol
Horns
Humans
Hypersensitivity
Immunoglobulin E
Interleukin-13
Interleukin-18
Interleukin-4
Keratinocytes
Lymph Nodes
Mast Cells
Mice
Models, Animal
Nerve Fibers
Nerve Growth Factor
Neurites
Semaphorin-3A
Semaphorins
Sensory Receptor Cells
Skin
Staphylococcus aureus
T-Lymphocytes
Th1 Cells
Xylitol
Olopatadine Hydrochloride
Dibenzoxepins
Enterotoxins
Farnesol
Immunoglobulin E
Interleukin-13
Interleukin-18
Interleukin-4
Nerve Growth Factor
Semaphorin-3A
Semaphorins
Xylitol
Figure
Cited by 1 articles
-
CSP0510 Lotion as a Novel Moisturizer Containing Citric Acid and Trisodium Phosphate Relieves Objective and Subjective Symptoms of Atopic Dermatitis
Ho-June Lee, Nam-Woong Yang, Jee-Young Choi, Jee-Bum Lee, Seung-Chul Lee
Ann Dermatol. 2016;28(3):344-351. doi: 10.5021/ad.2016.28.3.344.
Reference
-
1. Kanbara T, Nakamura K, Tanaka Y, Inoue Y, Tanaka K, Ikezawa Z. A study about the prevalence of infantile atopic dermatitis (the 5th report). Proceedings in Arerugi. 2009. 58:376.2. Katsuyama M, Wachi Y, Kitamura K, Suga C, Onuma S, Ikezawa Z. Correlation between the population of staphylococcus aureus on the skin and severity of score of dry type atopic dermatitis condition. Nippon Hifuka Gakkai Zasshi. 1997. 107:1103–1111.3. Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z. A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci. 2005. 38:197–205.4. Katsuyama M, Kobayashi Y, Ichikawa H, Mizuno A, Miyachi Y, Matsunaga K, Kawashima M. A novel method to control the balance of skin microflora Part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin. J Dermatol Sci. 2005. 38:207–213.5. Fujita H, Takahashi H, Aihara M, Hirasawa T, Ikezawa Z. Effects of bamboo leaf extract on atopic dermatitis model mice. J Environ Dermatol. 2006. 13:87–94.6. Terada M, Tsutsui H, Imai Y, Yasuda K, Mizutani H, Yamanishi K, Kubo M, Matsui K, Sano H, Nakanishi K. Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proc Natl Acad Sci U S A. 2006. 103:8816–8821.7. Kirino M, Kirino Y, Takeno M, Nagashima Y, Takahashi K, Kobayashi M, Murakami S, Hirasawa T, Ueda A, Aihara M, Ikezawa Z, Ishigatsubo Y. Heme oxygenase 1 attenuates the development of atopic dermatitis-like lesions in mice: implications for human disease. J Allergy Clin Immunol. 2008. 122:290–297. 7 e1-8.8. Matsukura S, Aihara M, Hirasawa T, Ikezawa Z. Effects of TNCB sensitization in DS-Nh mice, serving as a model of atopic dermatitis, in comparison with NC/Nga mice. Int Arch Allergy Immunol. 2005. 136:173–180.9. Yoshioka T, Hikita I, Matsutani T, Yoshida R, Asakawa M, Toyosaki-Maeda T, Hirasawa T, Suzuki R, Arimura A, Horikawa T. DS-Nh as an experimental model of atopic dermatitis induced by Staphylococcus aureus producing staphylococcal enterotoxin C. Immunology. 2003. 108:562–569.10. Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci. 2009. 53:48–54.11. Inoue Y, Aihara M, Harada I, Komori J, Kirino M, Ikezawa Z. Analysis of the pathogenesis by the quantitative measurement of interleukin-18 in the horny layer and Staphylococcus aureus colonization on the skin surface in patients with atopic dermatitis. Proceedings in Arerugi. 2009. 58:1262.12. Ikezawa Y. Exacerbative effect of cyclophosphamide on symptom of atopic dermatitis in DS-Nh mouse. Yokohama Medical Journal. 2004. 55:437–443.13. Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci. 2005. 39:105–112.14. Tatewaki S, Nakazawa M, Aihara M, Hongo N, Hotta Ch, Ikezawa Y, Takahashi K, Minami M, Ikezawa Z. Effects of cyclophosphamide on 2, 4-dinitrofluorobenzene-specific oral tolerance. J Environ Dermatol Cutan Allergol. 2007. 1:12–21.15. Yoshioka T, Hikita I, Asakawa M, Hirasawa T, Deguchi M, Matsutani T, Oku H, Horikawa T, Arimura A. Spontaneous scratching behaviour in DS-Nh mice as a possible model for pruritus in atopic dermatitis. Immunology. 2006. 118:293–301.16. Toyoda M, Nakamura M, Makino T, Hino T, Kagoura M, Morohashi M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002. 147:71–79.17. Pincelli C, Sevignani C, Manfredini R, Grande A, Fantini F, Bracci-Laudiero L, Aloe L, Ferrari S, Cossarizza A, Giannetti A. Expression and function of nerve growth factor and nerve growth factor receptor on cultured keratinocytes. J Invest Dermatol. 1994. 103:13–18.18. Botchkarev VA, Yaar M, Peters EM, Raychaudhuri SP, Botchkareva NV, Marconi A, Raychaudhuri SK, Paus R, Pincelli C. Neurotrophins in skin biology and pathology. J Invest Dermatol. 2006. 126:1719–1727.19. Pincelli C, Marconi A. Autocrine nerve growth factor in human keratinocytes. J Dermatol Sci. 2000. 22:71–79.20. Huang CH, Kuo IC, Xu H, Lee YS, Chua KY. Mite allergen induces allergic dermatitis with concomitant neurogenic inflammation in mouse. J Invest Dermatol. 2003. 121:289–293.21. Peters EM, Raap U, Welker P, Tanaka A, Matsuda H, Pavlovic-Masnicosa S, Hendrix S, Pincelli C. Neurotrophins act as neuroendocrine regulators of skin homeostasis in health and disease. Horm Metab Res. 2007. 39:110–124.22. Masahiko T. Useful therapeutic markers of atopic dermatitis: neurogenic factors. Skin Research. 2005. 4:Suppl 5. 87–93.23. Dou YC, Hagstromer L, Emtestam L, Johansson O. Increased nerve growth factor and its receptors in atopic dermatitis: an immunohistochemical study. Arch Dermatol Res. 2006. 298:31–37.24. Kanda N, Watanabe S. Histamine enhances the production of nerve growth factor in human keratinocytes. J Invest Dermatol. 2003. 121:570–577.25. Kojima M, Aihara M, Yamada M, Matsukura S, Hirasawa T, Ikezawa Z. Effects of neuropeptides in the development of the atopic dermatitis of mouse models. Allergol Int. 2004. 53:169–178.26. Izu K, Tokura Y. The various effects of four H1-antagonists on serum substance P levels in patients with atopic dermatitis. J Dermatol. 2005. 32:776–781.27. Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989. 337:362–364.28. Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, Brodie C, Renz H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur J Immunol. 1998. 28:3240–3251.29. Tamura T, Matsubara M, Takada C, Hasegawa K, Suzuki K, Ohmori K, Karasawa A. Effects of olopatadine hydrochloride, an antihistamine drug, on skin inflammation induced by repeated topical application of oxazolone in mice. Br J Dermatol. 2004. 151:1133–1142.30. Tamura T, Matsubara M, Hasegawa K, Ohmori K, Karasawa A. Olopatadine hydrochloride suppresses the rebound phenomenon after discontinuation of treatment with a topical steroid in mice with chronic contact hypersensitivity. Clin Exp Allergy. 2005. 35:97–103.31. Tamura T, Amano T, Ohmori K, Manabe H. The effects of olopatadine hydrochloride on the number of scratching induced by repeated application of oxazolone in mice. Eur J Pharmacol. 2005. 524:149–154.32. Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005. 6:789–800.33. Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008. 8:632–645.34. Yamaguchi J, Nakamura F, Aihara M, Yamashita N, Usui H, Hida T, Takei K, Nagashima Y, Ikezawa Z, Goshima Y. Semaphorin3A alleviates skin lesions and scratching behavior in NC/Nga mice, an atopic dermatitis model. J Invest Dermatol. 2008. 128:2842–2849.35. Tominaga M, Ogawa H, Takamori K. Decreased production of semaphorin 3A in the lesional skin of atopic dermatitis. Br J Dermatol. 2008. 158:842–844.36. Biro T, Toth BI, Marincsak R, Dobrosi N, Geczy T, Paus R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim Biophys Acta. 2007. 1772:1004–1021.37. Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995. 121:4309–4318.38. Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996. 383:525–528.39. Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998. 92:735–745.40. Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003. 3:159–167.41. Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DM, Nogueira AC, Dardenne M, Hermine O, Savino W. Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions. Proc Natl Acad Sci U S A. 2007. 104:5545–5550.42. Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, Boumsell L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J Immunol. 2001. 166:4348–4354.43. Mangasser-Stephan K, Dooley S, Welter C, Mutschler W, Hanselmann RG. Identification of human semaphorin E gene expression in rheumatoid synovial cells by mRNA differential display. Biochem Biophys Res Commun. 1997. 234:153–156.44. Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, Kikutani H. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002. 419:629–633.45. Bougeret C, Mansur IG, Dastot H, Schmid M, Mahouy G, Bensussan A, Boumsell L. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J Immunol. 1992. 148:318–323.46. Herold C, Bismuth G, Bensussan A, Boumsell L. Activation signals are delivered through two distinct epitopes of CD100, a unique 150 kDa human lymphocyte surface structure previously defined by BB18 mAb. Int Immunol. 1995. 7:1–8.47. Hall KT, Boumsell L, Schultze JL, Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM, Freeman GJ. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc Natl Acad Sci U S A. 1996. 93:11780–11785.48. Wu Y, Nadler MJ, Brennan LA, Gish GD, Timms JF, Fusaki N, Jongstra-Bilen J, Tada N, Pawson T, Wither J, Neel BG, Hozumi N. The B-cell transmembrane protein CD72 binds to and is an in vivo substrate of the protein tyrosine phosphatase SHP-1. Curr Biol. 1998. 8:1009–1017.49. Kumanogoh A, Watanabe C, Lee I, Wang X, Shi W, Araki H, Hirata H, Iwahori K, Uchida J, Yasui T, Matsumoto M, Yoshida K, Yakura H, Pan C, Parnes JR, Kikutani H. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000. 13:621–631.50. Watanabe C, Kumanogoh A, Shi W, Suzuki K, Yamada S, Okabe M, Yoshida K, Kikutani H. Enhanced immune responses in transgenic mice expressing a truncated form of the lymphocyte semaphorin CD100. J Immunol. 2001. 167:4321–4328.51. Holmes S, Downs AM, Fosberry A, Hayes PD, Michalovich D, Murdoch P, Moores K, Fox J, Deen K, Pettman G, Wattam T, Lewis C. Sema7A is a potent monocyte stimulator. Scand J Immunol. 2002. 56:270–275.52. Kurschat P, Bielenberg D, Rossignol-Tallandier M, Stahl A, Klagsbrun M. Neuron restrictive silencer factor NRSF/REST is a transcriptional repressor of neuropilin-1 and diminishes the ability of semaphorin 3A to inhibit keratinocyte migration. J Biol Chem. 2006. 281:2721–2729.53. Tordjman R, Lepelletier Y, Lemarchandel V, Cambot M, Gaulard P, Hermine O, Romeo PH. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat Immunol. 2002. 3:477–482.54. Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, Shirvan A, Barzilai A, Hermine O. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006. 36:1782–1793.55. Takano N, Arai I, Kurachi M. Analysis of the spontaneous scratching behavior by NC/Nga mice: a possible approach to evaluate antipruritics for subjects with atopic dermatitis. Eur J Pharmacol. 2003. 471:223–228.56. Takano N, Sakurai T, Kurachi M. Effects of anti-nerve growth factor antibody on symptoms in the NC/Nga mouse, an atopic dermatitis model. J Pharmacol Sci. 2005. 99:277–286.57. Pond A, Roche FK, Letourneau PC. Temporal regulation of neuropilin-1 expression and sensitivity to semaphorin 3A in NGF- and NT3-responsive chick sensory neurons. J Neurobiol. 2002. 51:43–53.58. Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995. 14:949–959.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Semaphorin 3A and Neuropilin 1 in Asthma
- Characteristic Clinical Features of Korean Atopic Dermatitis Patients with Interleukin-17 Receptor A Gene Mutation
- Interleukin-4 as a New Index of Disease Severity in Atopic Dermatitis
- Role of Chemokines in the Pathogenesis of Atopic Dermatitis
- Expression of Genetic Polymorphism of Interleukin-10 (IL-10) and Tumor Necrosis Factor-beta (TNF-beta) in Patients with Atopic Dermatitis