Allergy Asthma Immunol Res.
2013 Nov;5(6):402-408. 10.4168/aair.2013.5.6.402.
Effect of Cholesterol Depletion on Interleukin-8 Production in Human Respiratory Epithelial Cells
- Affiliations
-
- 1Department of Pediatrics and Institute of Allergy, Severance Medical Research Institute, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. kekim@yuhs.ac
- KMID: 2260285
- DOI: http://doi.org/10.4168/aair.2013.5.6.402
Abstract
- PURPOSE
The lipid entities of cell membranes are components of the immune system and important mediators of inflammation. Despite increasing interest in the function of epithelial cells in inflammation, the role of cholesterol in this process has not been described. Here, we investigated the effect of cholesterol depletion on the inflammatory process in airway epithelial cells via the expression of interleukin (IL)-8 as a marker of inflammation.
METHODS
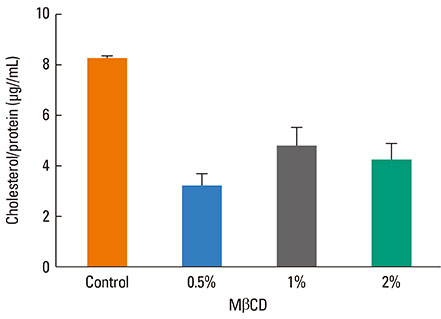
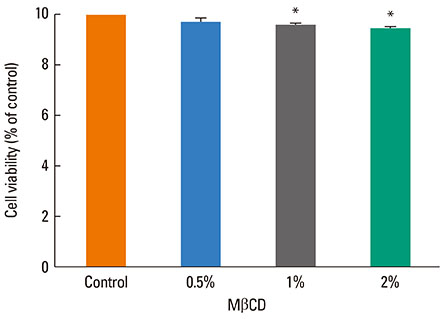
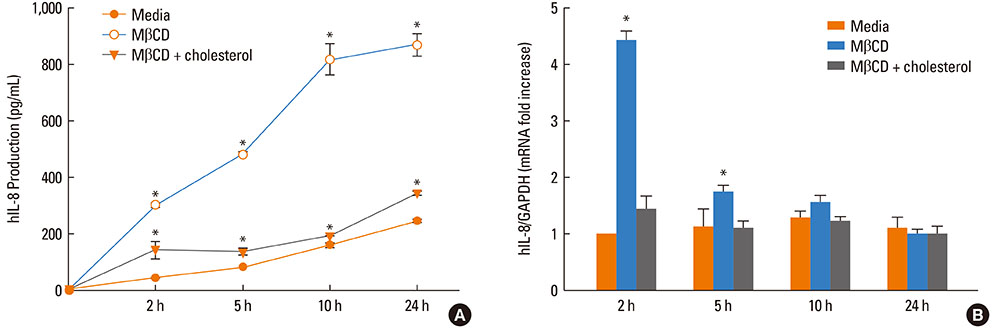
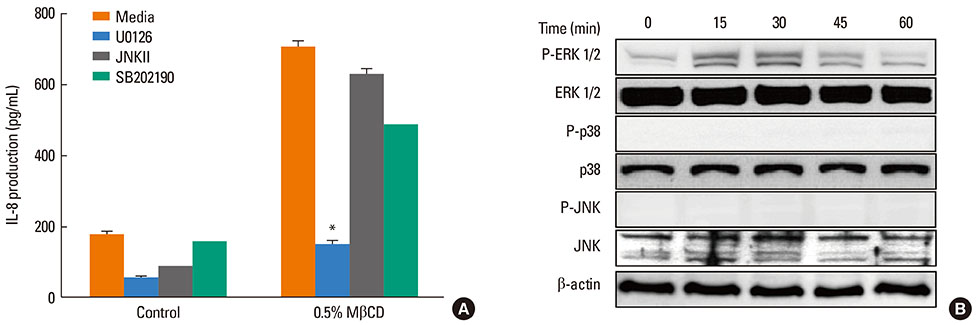
A 549 cells were treated with 0.5% methyl-beta-cyclodextrin as a selective cholesterol extractor. The IL-8 level was assessed by enzyme-linked immunosorbent assay and reassessed after cholesterol repletion. Mitogen-activated protein kinase (MAPK) inhibitors were used to determine the upstream signaling pathway for IL-8 production in cholesterol-depleted cells.
RESULTS
We found a relationship between the amount of cholesterol in A 549 cells and inflammation of the airway. IL-8 production was increased in cholesterol-depleted A 549 cells and restored by cholesterol repletion. IL-8 production was decreased by pretreatment with the extracellular signal-regulated kinase (ERK) inhibitor U0126 but not with JNK inhibitor II or the p38 MAPK inhibitor SB202190.
CONCLUSIONS
Our findings suggest that inflammatory responses are increased in cholesterol-depleted epithelial cells via the MAPK signaling system, predominantly by the ERK pathway. We conclude that the lipid components of airwayepithelial cells may play a role in the inflammatory process.
MeSH Terms
-
beta-Cyclodextrins
Butadienes
Cell Membrane
Cholesterol
Enzyme-Linked Immunosorbent Assay
Epithelial Cells
Humans
Immune System
Inflammation
Inflammation Mediators
Interleukin-8
Interleukins
MAP Kinase Signaling System
Nitriles
p38 Mitogen-Activated Protein Kinases
Phosphotransferases
Protein Kinases
Butadienes
Cholesterol
Inflammation Mediators
Interleukin-8
Interleukins
Nitriles
Phosphotransferases
Protein Kinases
beta-Cyclodextrins
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Proud D, Leigh R. Epithelial cells and airway diseases. Immunol Rev. 2011; 242:186–204.2. Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011; 242:205–219.3. Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003; 44:655–667.4. Chang TH, Segovia J, Sabbah A, Mgbemena V, Bose S. Cholesterol-rich lipid rafts are required for release of infectious human respiratory syncytial virus particles. Virology. 2012; 422:205–213.5. Saeki K, Fukuyama S, Ayada T, Nakaya M, Aki D, Takaesu G, Hanada T, Matsumura Y, Kobayashi T, Nakagawa R, Yoshimura A. A major lipid raft protein raftlin modulates T cell receptor signaling and enhances Th17-mediated autoimmune responses. J Immunol. 2009; 182:5929–5937.6. San-Juan-Vergara H, Sampayo-Escobar V, Reyes N, Cha B, Pacheco-Lugo L, Wong T, Peeples ME, Collins PL, Castaño ME, Mohapatra SS. Cholesterol-rich microdomains as docking platforms for respiratory syncytial virus in normal human bronchial epithelial cells. J Virol. 2012; 86:1832–1843.7. Abraham SN, Duncan MJ, Li G, Zaas D. Bacterial penetration of the mucosal barrier by targeting lipid rafts. J Investig Med. 2005; 53:318–321.8. Strieter RM. Interleukin-8: a very important chemokine of the human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2002; 283:L688–L689.9. Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998; 9:9–23.10. Hong JY, Lee KE, Kim KW, Sohn MH, Kim KE. Chitinase induce the release of IL-8 in human airway epithelial cells, via Ca2+-dependent PKC and ERK pathways. Scand J Immunol. 2010; 72:15–21.11. Lee KE, Kim JW, Jeong KY, Kim KE, Yong TS, Sohn MH. Regulation of German cockroach extract-induced IL-8 expression in human airway epithelial cells. Clin Exp Allergy. 2007; 37:1364–1373.12. Sohn MH, Lee KE, Kim KW, Kim ES, Park JY, Kim KE. Calciumcalmodulin mediates house dust mite-induced ERK activation and IL-8 production in human respiratory epithelial cells. Respiration. 2007; 74:447–453.13. Sohn MH, Lee KE, Choi SY, Kwon BC, Chang MW, Kim KE. Effect of Mycoplasma pneumoniae lysate on interleukin-8 gene expression in human respiratory epithelial cells. Chest. 2005; 128:322–326.14. Pontes Soares C, Portilho DM, da Silva Sampaio L, Einicker-Lamas M, Morales MM, Costa ML, Dos Santos Mermelstein C. Membrane cholesterol depletion by methyl-beta-cyclodextrin enhances the expression of cardiac differentiation markers. Cells Tissues Organs. 2010; 192:187–199.15. Kabouridis PS, Janzen J, Magee AL, Ley SC. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur J Immunol. 2000; 30:954–963.16. Chen X, Resh MD. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J Biol Chem. 2002; 277:49631–49637.17. Mathay C, Pierre M, Pittelkow MR, Depiereux E, Nikkels AF, Colige A, Poumay Y. Transcriptional profiling after lipid raft disruption in keratinocytes identifies critical mediators of atopic dermatitis pathways. J Invest Dermatol. 2011; 131:46–58.18. Hackett TL, Knight DA. The role of epithelial injury and repair in the origins of asthma. Curr Opin Allergy Clin Immunol. 2007; 7:63–68.19. Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007; 120:1233–1244.20. Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc. 2009; 6:678–682.21. Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000; 105:193–204.22. Swindle EJ, Collins JE, Davies DE. Breakdown in epithelial barrier function in patients with asthma: identification of novel therapeutic approaches. J Allergy Clin Immunol. 2009; 124:23–34.23. Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008; 121:5–10.24. Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, Mukhopadhyay S, Smith GD, Palmer CN, McLean WH, Irvine AD. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008; 121:872–877.e9.25. Marenholz I, Nickel R, Rüschendorf F, Schulz F, Esparza-Gordillo J, Kerscher T, Grüber C, Lau S, Worm M, Keil T, Kurek M, Zaluga E, Wahn U, Lee YA. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. J Allergy Clin Immunol. 2006; 118:866–871.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Corticosteroids on IL-6, IL-8 and RANTES Production from RSV-infected Epithelial Cells
- The Effect of Dexamethasone on Respiratory Syncytial Virus Induced RANTES and IL-8 Production of a Human Bronchial Epithelial Cell Line
- Cholesterol Depletion in Cell Membranes of Human Airway Epithelial Cells Suppresses MUC5AC Gene Expression
- Effects of Scutellarin on MUC5AC Mucin Production Induced by Human Neutrophil Elastase or Interleukin 13 on Airway Epithelial Cells
- Effect of Mycoplasma pneumoniae on IL-8 Gene Expression in Human Respiratory Epithelial Cells