Allergy Asthma Immunol Res.
2014 Nov;6(6):504-510. 10.4168/aair.2014.6.6.504.
Reference Ranges and Determinant Factors for Exhaled Nitric Oxide in a Healthy Korean Elderly Population
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. shcho@snu.ac.kr
- 3Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul, Korea.
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 8Department of Internal Medicine, Eulji University College of Medicine, Seoul, Korea.
- 9Department of Internal Medicine, Ajou University College of Medicine, Suwon, Korea.
- 10Department of Preventive Medicine, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2260172
- DOI: http://doi.org/10.4168/aair.2014.6.6.504
Abstract
- PURPOSE
Exhaled nitric oxide (NO) is a useful non-invasive biomarker for asthma diagnosis; however, the literature suggests that exhaled NO levels may be affected by demographic factors. The present analysis investigated determinant factors that present exhaled NO reference levels for Korean elderly adults.
METHODS
For reference levels, we analyzed the baseline data of healthy adult participants in the Ansung cohort. The fraction of exhaled NO (FeNO) was measured by NIOX MINO(R). The characterization of the subjects was performed through structured questionnaires, spirometry, and methacholine challenge tests. To validate the diagnostic utility of the determined reference levels, asthma patients were recruited from medical institutions for FeNO measurement.
RESULTS
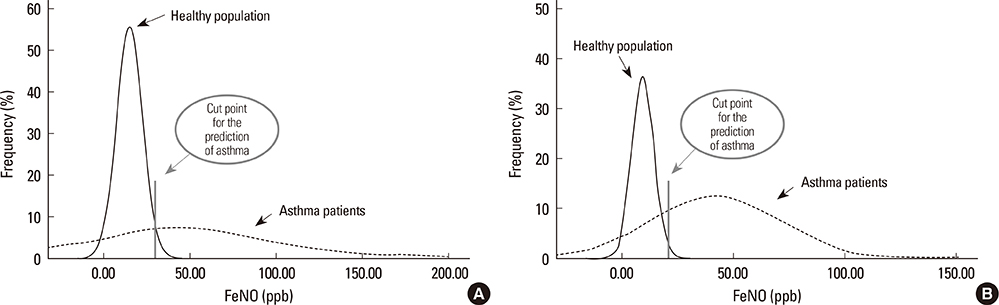
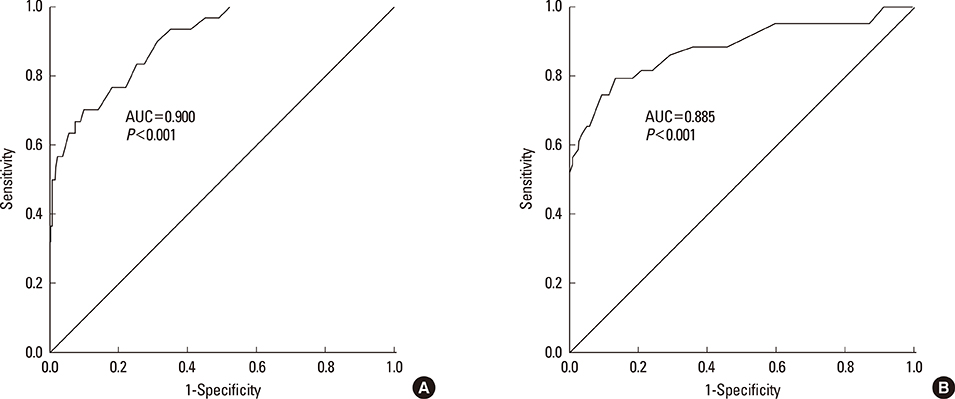
A total of 570 healthy subjects were analyzed (mean age, 59.9+/-12.3; male, 37.0%) for reference levels. FeNO levels significantly correlated with weight, height, body mass index, atopy, or forced expiratory volume in 1 second % predicted by simple linear regression analysis. Multiple linear regression analysis identified gender as an independent determinant for FeNO levels; subsequently, the reference values for FeNO were 18.2+/-10.6 ppb (5th to 95th percentile, 6.0 to 37.4 ppb) for males and 12.1+/-6.9 ppb (5th to 95th percentile, 2.5 to 27.0 ppb) for females. The diagnostic utility of FeNO reference levels was validated by receiver operating curve analysis (area under curve, 0.900 for males and 0.885 for females) for diagnosing asthma. The optimal cutoff values for the prediction of asthma were 30.5 ppb for males and 20.5 ppb for females.
CONCLUSIONS
The current analysis presented reference ranges and the diagnostic utility of FeNO levels for asthma in Korean elderly adults.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Reference ranges for induced sputum eosinophil counts in Korean adult population
Mi-Yeong Kim, Eun-Jung Jo, Seung-Eun Lee, Suh-Young Lee, Woo-Jung Song, Tae-Wan Kim, Gyu-Young Hur, Jae-Hyung Lee, Tae-Bum Kim, Heung-Woo Park, Yoon-Seok Chang, Hae-Sim Park, Kyung-Up Min, Sang-Heon Cho
Asia Pac Allergy. 2014;4(3):149-155. doi: 10.5415/apallergy.2014.4.3.149.The KAAACI Standardization Committee Report on the procedure and application of fractional exhaled nitric oxide measurement
Jae-Woo Kwon, Woo-Jung Song, Min-Hye Kim, Kyung-Hwan Lim, Min-Suk Yang, Jae-Woo Jung, Jeongmin Lee, Dong In Suh, Yoo Seob Shin, Sae-Hoon Kim, Sang-Heon Kim, Byung-Jae Lee, Sang-Heon Cho
Allergy Asthma Respir Dis. 2017;5(4):185-192. doi: 10.4168/aard.2017.5.4.185.
Reference
-
1. National Heart, Lung, and Blood Institute, National Institutes of Health. International consensus report on diagnosis and treatment of asthma Bethesda, Maryland 20892. Publication no 92-3091, March 1992. Eur Respir J. 1992; 5:601–641.2. Anderson SD, Brannan J, Spring J, Spalding N, Rodwell LT, Chan K, Gonda I, Walsh A, Clark AR. A new method for bronchial-provocation testing in asthmatic subjects using a dry powder of mannitol. Am J Respir Crit Care Med. 1997; 156:758–765.3. Hunter CJ, Brightling CE, Woltmann G, Wardlaw AJ, Pavord ID. A comparison of the validity of different diagnostic tests in adults with asthma. Chest. 2002; 121:1051–1057.4. Maestrelli P, Saetta M, Di Stefano A, Calcagni PG, Turato G, Ruggieri MP, Roggeri A, Mapp CE, Fabbri LM. Comparison of leukocyte counts in sputum, bronchial biopsies, and bronchoalveolar lavage. Am J Respir Crit Care Med. 1995; 152:1926–1931.5. Kharitonov SA, Gonio F, Kelly C, Meah S, Barnes PJ. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J. 2003; 21:433–438.6. Barnes PJ, Belvisi MG. Nitric oxide and lung disease. Thorax. 1993; 48:1034–1043.7. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.8. Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, Ligabue G, Ciaccia A, Saetta M, Papi A. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003; 167:418–424.9. Kharitonov SA, Yates D, Barnes PJ. Increased nitric oxide in exhaled air of normal human subjects with upper respiratory tract infections. Eur Respir J. 1995; 8:295–297.10. de Gouw HW, Grünberg K, Schot R, Kroes AC, Dick EC, Sterk PJ. Relationship between exhaled nitric oxide and airway hyperrehyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur Respir J. 1998; 11:126–132.11. Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today. 1995; 16:128–130.12. Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998; 53:91–95.13. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.14. Sandrini A, Taylor DR, Thomas PS, Yates DH. Fractional exhaled nitric oxide in asthma: an update. Respirology. 2010; 15:57–70.15. Kim SH, Kim TH, Sohn JW, Yoon HJ, Shin DH, Park SS. Reference values and determinants of exhaled nitric oxide in healthy Korean adults. J Asthma. 2010; 47:563–567.16. Shin C, Abbott RD, Lee H, Kim J, Kimm K. Prevalence and correlates of orthostatic hypotension in middle-aged men and women in Korea: the Korean Health and Genome Study. J Hum Hypertens. 2004; 18:717–723.17. Morris JF. Spirometry in the evaluation of pulmonary function. West J Med. 1976; 125:110–118.18. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–329.19. Tsang KW, Ip SK, Leung R, Tipoe GL, Chan SL, Shum IH, Ip MS, Yan C, Fung PC, Chan-Yeung M, Lam W. Exhaled nitric oxide: the effects of age, gender and body size. Lung. 2001; 179:83–91.20. Olivieri M, Talamini G, Corradi M, Perbellini L, Mutti A, Tantucci C, Malerba M. Reference values for exhaled nitric oxide (reveno) study. Respir Res. 2006; 7:94.21. Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, Weatherall M, Beasley R. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med. 2007; 176:238–242.22. Ko FW, Leung TF, Wong GW, Chu JH, Sy HY, Hui DS. Determinants of, and reference equation for, exhaled nitric oxide in the Chinese population. Eur Respir J. 2013; 42:767–775.23. Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999; 159:69–73.24. Wong GW, Liu EK, Leung TF, Yung E, Ko FW, Hui DS, Fok TF, Lai CK. High levels and gender difference of exhaled nitric oxide in Chinese schoolchildren. Clin Exp Allergy. 2005; 35:889–893.25. Latzin P, Beck J, Griese M. Exhaled nitric oxide in healthy children: variability and a lack of correlation with atopy. Pediatr Allergy Immunol. 2002; 13:37–46.26. Kovesi T, Kulka R, Dales R. Exhaled nitric oxide concentration is affected by age, height, and race in healthy 9- to 12-year-old children. Chest. 2008; 133:169–175.27. Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005; 115:1130–1136.28. Olin AC, Bake B, Torén K. Fraction of exhaled nitric oxide at 50 mL/s: reference values for adult lifelong never-smokers. Chest. 2007; 131:1852–1856.29. Chng SY, Van Bever HP, Lian D, Lee SX, Xu XN, Wang XS, Goh DY. Relationship between exhaled nitric oxide and atopy in Asian young adults. Respirology. 2005; 10:40–45.30. Olin AC, Aldenbratt A, Ekman A, Ljungkvist G, Jungersten L, Alving K, Torén K. Increased nitric oxide in exhaled air after intake of a nitrate-rich meal. Respir Med. 2001; 95:153–158.31. Yemaneberhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997; 350:85–90.32. Ernst P, Cormier Y. Relative scarcity of asthma and atopy among rural adolescents raised on a farm. Am J Respir Crit Care Med. 2000; 161:1563–1566.33. Lee SY, Kwon JW, Seo JH, Song YH, Kim BJ, Yu J, Park KS, Kim H, Kim EJ, Lee JS, Hong SJ. Prevalence of atopy and allergic diseases in Korean children: associations with a farming environment and rural lifestyle. Int Arch Allergy Immunol. 2012; 158:168–174.34. Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, Falsey AR, Mathur SK, Ramsdell JW, Rogers L, Stempel DA, Lima JJ, Fish JE, Wilson SR, Boyd C, Patel KV, Irvin CG, Yawn BP, Halm EA, Wasserman SI, Sands MF, Ershler WB, Ledford DK. Asthma in Elderly workshop participants. Asthma in the elderly: current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011; 128:S4–24.35. Kim YK, Kim SH, Tak YJ, Jee YK, Lee BJ, Kim SH, Park HW, Jung JW, Bahn JW, Chang YS, Choi DC, Chang SI, Min KU, Kim YY, Cho SH. High prevalence of current asthma and active smoking effect among the elderly. Clin Exp Allergy. 2002; 32:1706–1712.36. King MJ, Hanania NA. Asthma in the elderly: current knowledge and future directions. Curr Opin Pulm Med. 2010; 16:55–59.37. Enright PL, McClelland RL, Newman AB, Gottlieb DJ, Lebowitz MD. Cardiovascular Health Study Research Group. Underdiagnosis and undertreatment of asthma in the elderly. Chest. 1999; 116:603–613.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurements of Exhaled Nitric Oxide in Newborns
- Measurement and Interpretation of Fractional Exhaled Nitric Oxide
- Measurements of fractional exhaled nitric oxide in pediatric asthma
- The Usefulness of Exhaled Nitric Oxide Test in Exercise-Induced Bronchoconstriction
- Utility of Fractional Exhaled Nitric Oxide in the Diagnosis of Asthma and the Assessment of Asthma Control