Korean J Neurotrauma.
2014 Oct;10(2):119-122. 10.13004/kjnt.2014.10.2.119.
Use of Annular Closure Device (Barricaid(R)) for Preventing Lumbar Disc Reherniation: One-Year Results of Three Cases
- Affiliations
-
- 1Department of Neurosurgery, Spine and Spinal Cord Research Institute, Yonsei University College of Medicine, Seoul, Korea. shindongah@me.com
- KMID: 2256244
- DOI: http://doi.org/10.13004/kjnt.2014.10.2.119
Abstract
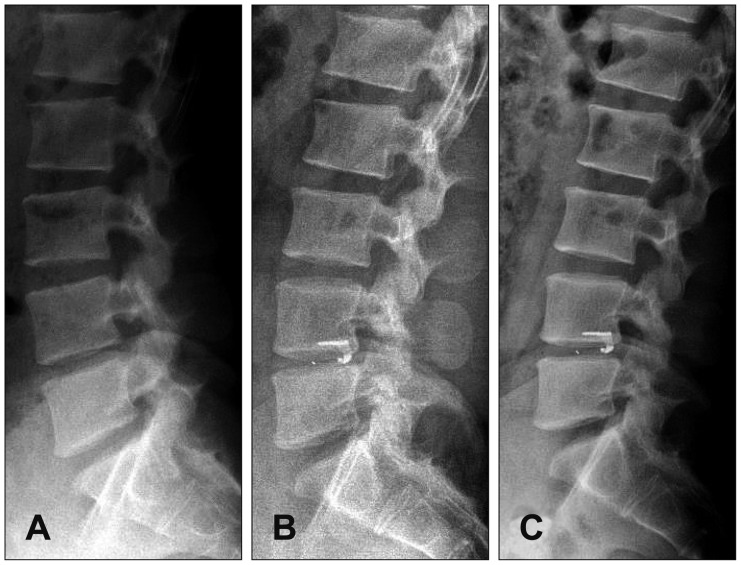
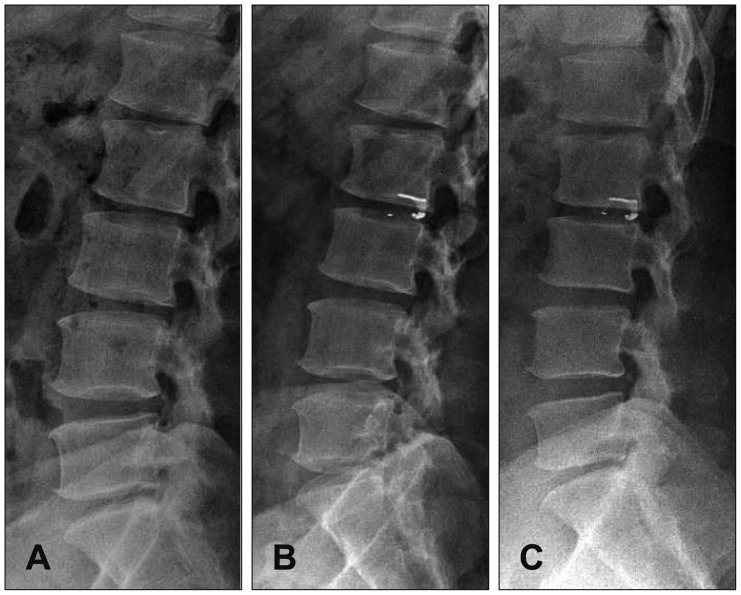
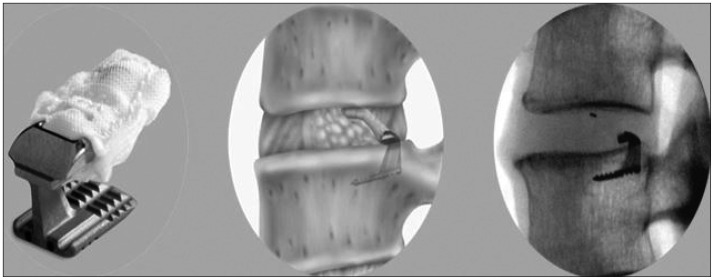
- Although lumbar discectomy is an effective treatment for lumbar disc herniation, complications exist, including postoperative disc height loss, facet joint degeneration, and recurrent disc herniation. To solve these problems, annular closure devices have been utilized in other countries, producing satisfactory results, but there has been no report of annular closure device use in our country. Here, we demonstrate the preliminary reports of Barricaid(R) insertion in 3 patients who underwent surgery for lumbar disc herniation.
Figure
Reference
-
1. Bouma GJ, Barth M, Ledic D, Vilendecic M. The high-risk discectomy patient: prevention of reherniation in patients with large anular defects using an anular closure device. Eur Spine J. 2013; 22:1030–1036. PMID: 23377540.
Article2. Carragee EJ, Han MY, Suen PW, Kim D. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am. 2003; 85-A:102–108. PMID: 12533579.3. Mobbs RJ, Newcombe RL, Chandran KN. Lumbar discectomy and the diabetic patient: incidence and outcome. J Clin Neurosci. 2001; 8:10–13. PMID: 11320971.
Article4. Parker SL, Grahovac G, Vukas D, Vilendecic M, Ledic D, McGirt MJ, et al. Effect of An Annular Closure Device (Barricaid) on Same Level Recurrent Disc Herniation and Disc Height Loss After Primary Lumbar Discectomy: Two-Year Results of a Multi-Center Prospective Cohort Study. J Spinal Disord Tech. 2013.5. Shimia M, Babaei-Ghazani A, Sadat BE, Habibi B, Habibzadeh A. Risk factors of recurrent lumbar disk herniation. Asian J Neurosurg. 2013; 8:93–96. PMID: 24049552.
Article6. Suk KS, Lee HM, Moon SH, Kim NH. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976). 2001; 26:672–676. PMID: 11246384.7. Swartz KR, Trost GR. Recurrent lumbar disc herniation. Neurosurg Focus. 2003; 15:E10. PMID: 15347228.
Article8. Trummer M, Eustacchio S, Barth M, Klassen PD, Stein S. Barricaid annular closure device reduces risk of facet degeneration. Clin Neurol Neurosurg. 2013; 115:1440–1445. PMID: 23473658.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Limited Lumbar Discectomy with an Annulus Closure Device: One-Year Clinical and Radiographic Results from a Prospective, Multi-Center Study
- Efficacy of a Novel Annular Closure Device after Lumbar Discectomy in Korean Patients: A 24-Month Follow-Up of a Randomized Controlled Trial
- Contralateral Reherniation after Open Lumbar Microdiscectomy : A Comparison with Ipsilateral Reherniation
- Rapid Repeated Recurrent Lumbar Disc Herniation after Microscopic Discectomies
- Discographic Findings in the Lumbar Disc Herniations