J Breast Cancer.
2012 Jun;15(2):162-171. 10.4048/jbc.2012.15.2.162.
Overexpression of Cell Cycle Progression Inhibitor Geminin is Associated with Tumor Stem-Like Phenotype of Triple-Negative Breast Cancer
- Affiliations
-
- 1INT Fondazione Pascale, Naples, Italy. monicantile@libero.it
- KMID: 2242186
- DOI: http://doi.org/10.4048/jbc.2012.15.2.162
Abstract
- PURPOSE
Triple-negative breast cancer, has a significant clinical relevance being associated with a shorter median time to relapse and death and does not respond to endocrine therapy or other available targeted agents. For this reason, identifying the molecular pathways associated with increased aggressiveness, for example the presence of stem cell populations within the tumor and alteration of genes associated with cell cycle regulation represents an important objective in the clinical research into this neoplasm.
METHODS
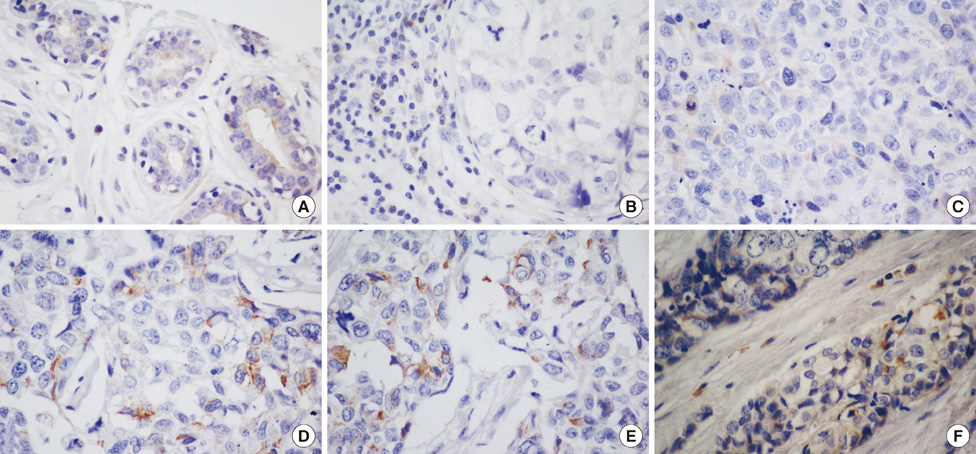
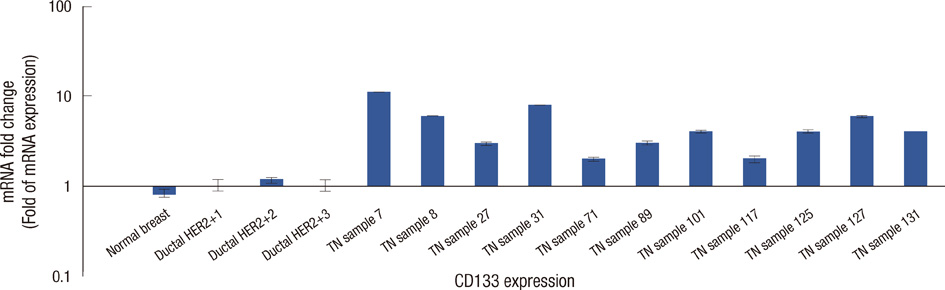
To investigate the role of cell cycle progression inhibitor Geminin in triple-negative breast cancers and its potential correlation with stem-like phenotype of this neoplasm, we used tissue microarray technology to build a specific triple-negative breast cancer tissue micro-array. Geminin and cancer stem cell marker CD133 expression was further investigated at the mRNA level for selected breast tumor samples through realtime polymerase chain reaction quantification.
RESULTS
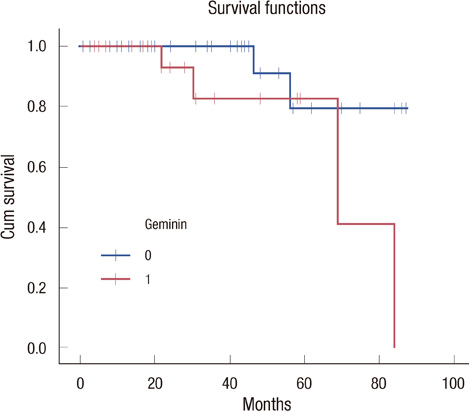
Our results showed that CD133 expression was significantly associated to high Geminin expression (p=0.017), a strong association between Ki-67 and tumor grade (p=0.020) and an inverse association between Geminin expression and lymphonode metastases (p=0.058), and a trend of statistically significance between Geminin marker expression and survival of triple-negative breast cancer patients (p=0.076).
CONCLUSION
The strong association between the expression of CD133 and Geminin could be useful in molecular stratification of breast tumors and in particular of triple-negative breast cancers.
MeSH Terms
Figure
Cited by 1 articles
-
Emerging Role of Geminin as a Prognostic Marker in Systemic Malignancies
Shailendra Kapoor
J Breast Cancer. 2012;15(4):481-481. doi: 10.4048/jbc.2012.15.4.481.
Reference
-
1. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001. 98:10869–10874.
Article2. van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002. 347:1999–2009.
Article3. Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008. 14:1368–1376.
Article4. Chacón RD, Costanzo MV. Triple-negative breast cancer. Breast Cancer Res. 2010. 12:Suppl 2. S3.
Article5. Rakha EA, El-Sayed ME, Reis-Filho J, Ellis IO. Patho-biological aspects of basal-like breast cancer. Breast Cancer Res Treat. 2009. 113:411–422.
Article6. Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010. 23:123–133.
Article7. Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007. 26:2126–2132.
Article8. Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, et al. The CD44+/CD24- phenotype is enriched in basallike breast tumors. Breast Cancer Res. 2008. 10:R53.
Article9. Arnes JB, Collett K, Akslen LA. Independent prognostic value of the basal-like phenotype of breast cancer and associations with EGFR and candidate stem cell marker BMI-1. Histopathology. 2008. 52:370–380.
Article10. Chaffer CL, Weinberg RA. Cancer cell of origin: spotlight on luminal progenitors. Cell Stem Cell. 2010. 7:271–272.
Article11. Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009. 69:1302–1313.
Article12. Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008. 3:e2888.
Article13. Takeda DY, Dutta A. DNA replication and progression through S phase. Oncogene. 2005. 24:2827–2843.
Article14. Gonzalez MA, Tachibana KE, Chin SF, Callagy G, Madine MA, Vowler SL, et al. Geminin predicts adverse clinical outcome in breast cancer by reflecting cell-cycle progression. J Pathol. 2004. 204:121–130.
Article15. Dudderidge TJ, Stoeber K, Loddo M, Atkinson G, Fanshawe T, Griffiths DF, et al. Mcm2, Geminin, and KI67 define proliferative state and are prognostic markers in renal cell carcinoma. Clin Cancer Res. 2005. 11:2510–2517.
Article16. Dudderidge TJ, McCracken SR, Loddo M, Fanshawe TR, Kelly JD, Neal DE, et al. Mitogenic growth signalling, DNA replication licensing, and survival are linked in prostate cancer. Br J Cancer. 2007. 96:1384–1393.
Article17. Vargas PA, Cheng Y, Barrett AW, Craig GT, Speight PM. Expression of Mcm-2, Ki-67 and geminin in benign and malignant salivary gland tumours. J Oral Pathol Med. 2008. 37:309–318.
Article18. Shrestha P, Saito T, Hama S, Arifin MT, Kajiwara Y, Yamasaki F, et al. Geminin: a good prognostic factor in high-grade astrocytic brain tumors. Cancer. 2007. 109:949–956.
Article19. Zhu W, Depamphilis ML. Selective killing of cancer cells by suppression of geminin activity. Cancer Res. 2009. 69:4870–4877.
Article20. Shomori K, Nishihara K, Tamura T, Tatebe S, Horie Y, Nosaka K, et al. Geminin, Ki67, and minichromosome maintenance 2 in gastric hyperplastic polyps, adenomas, and intestinal-type carcinomas: pathobiological significance. Gastric Cancer. 2010. 13:177–185.
Article21. Torres-Rendon A, Roy S, Craig GT, Speight PM. Expression of Mcm2, geminin and Ki67 in normal oral mucosa, oral epithelial dysplasias and their corresponding squamous-cell carcinomas. Br J Cancer. 2009. 100:1128–1134.
Article22. Montanari M, Boninsegna A, Faraglia B, Coco C, Giordano A, Cittadini A, et al. Increased expression of geminin stimulates the growth of mammary epithelial cells and is a frequent event in human tumors. J Cell Physiol. 2005. 202:215–222.
Article23. Graeser M, McCarthy A, Lord CJ, Savage K, Hills M, Salter J, et al. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2010. 16:6159–6168.
Article24. Mimeault M, Batra SK. New promising drug targets in cancer- and metastasis-initiating cells. Drug Discov Today. 2010. 15:354–364.
Article25. Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009. 106:13820–13825.
Article26. Wu Y, Wu PY. CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev. 2009. 18:1127–1134.27. Cho DY, Lin SZ, Yang WK, Hsu DM, Lin HL, Lee HC, et al. The role of cancer stem cells (CD133(+)) in malignant gliomas. Cell Transplant. 2011. 20:121–125.28. Liu Q, Li JG, Zheng XY, Jin F, Dong HT. Expression of CD133, PAX2, ESA, and GPR30 in invasive ductal breast carcinomas. Chin Med J (Engl). 2009. 122:2763–2769.
Article29. Zhao P, Lu Y, Jiang X, Li X. Clinicopathological significance and prognostic value of CD133 expression in triple-negative breast carcinoma. Cancer Sci. 2011. 102:1107–1111.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LncRNA DLG1-AS1 Promotes Cancer Cell Proliferation in Triple Negative Breast Cancer by Downregulating miR-203
- Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity
- Comment to “Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosisâ€
- The expression of Rab5 and its effect on invasion, migration and exosome secretion in triple negative breast cancer
- Inhibitor of DNA-binding 4 contributes to the maintenance and expansion of cancer stem cells in 4T1 mouse mammary cancer cell line