J Breast Cancer.
2009 Sep;12(3):163-169. 10.4048/jbc.2009.12.3.163.
Preoperative Axillary Staging Using 18F-FDG PET/CT and Ultrasonography in Breast Cancer Patients
- Affiliations
-
- 1Department of Surgery, Research Institute for Medical Science, Chungnam National University School of Medicine, Daejeon, Korea. eschang@cnu.ac.kr
- KMID: 2242007
- DOI: http://doi.org/10.4048/jbc.2009.12.3.163
Abstract
- PURPOSE
The axillary lymph node status is an important prognostic factor for recurrence and survival of patients who have primary breast cancer. This study determined the accuracy of ultrasonography and 18F-FDG positron emission tomography (PET)/computed tomography (CT) in preoperative staging in axilla in patients with breast cancer.
METHODS
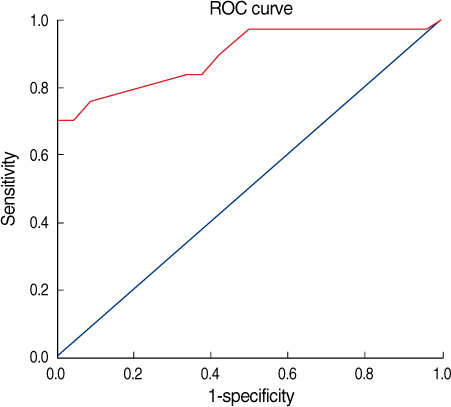
One hundred seventy-one patients with primary breast cancer were recruited from January 2007 to August 2008. All the patients underwent axillary ultrasonography and 18F-FDG PET/CT for the axillary staging before their operation. RESULTS: The overall sensitivity, specificity, and the positive and negative predictive values and the accuracy of axillary ultrasonography for making the diagnosis of axillary metastasis were 73.07%, 84.87%, 67.85%, 87.82%, and 81.28%, respectively. On a visual assessment of 18F-FDG PET/CT, the diagnostic accuracy was 85.38% with 69.23% sensitivity, 92.43% specificity, a positive predictive value of 80.00%, and a negative predictive value of 87.30%. By the combined use axillary ultrasonography and 18F-FDG PET/CT to the axilla, the sensitivity, specificity, the positive and negative predictive values and the diagnostic accuracy were 82.35%, 97.91%, 93.33%, 94.00%, and 93.84%, respectively.
CONCLUSION
The combination of 18F-FDG PET/CT and ultrasonography improves preoperative axillary staging in breast cancer that are often not found if only one imaging modalities are applied.
MeSH Terms
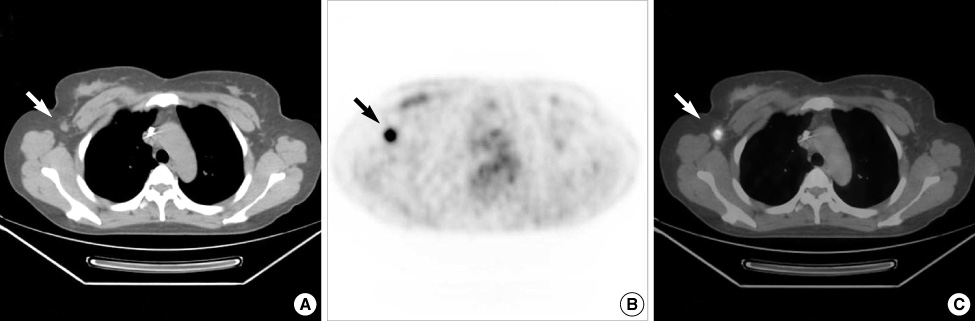
Figure
Reference
-
1. McCready DR, Hortobagyi GN, Kau SW, Smith TL, Buzdar AU, Balch CM. The prognostic significance of lymph node metastases after preoperative chemotherapy for locally advanced breast cancer. Arch Surg. 1989. 124:21–25.
Article2. Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER. Relation of the number of positive axillary nodes to the prognosis of patients with primary breast cancer. Cancer. 1983. 52:1551–1557.
Article3. Henderson IC, Canellos GP. Cancer of the breast: the past decade (first of two parts). N Engl J Med. 1980. 302:17–30.4. Kwan W, Jackson J, Weir LM, Dingee C, McGregor G, Olivotto IA. Chronic arm morbidity after curative breast cancer treatment: prevalence and impact on quality of life. J Clin Oncol. 2002. 20:4242–4248.
Article5. Aarsvold JN, Alazraki NP. Update on detection of sentinel lymph nodes in patients with breast cancer. Semin Nucl Med. 2005. 35:116–128.
Article6. Nori J, Vanzi E, Bazzocchi M, Bufalini FN, Distante V, Branconi F, et al. Role of axillary ultrasound examination in the selection of breast cancer patients for sentinel node biopsy. Am J Surg. 2007. 193:16–20.
Article7. Sato K, Tamaki K, Tsuda H, Kosuda S, Kusano S, Hiraide H, et al. Utility of axillary ultrasound examination to select breast cancer patients suited for optimal sentinel node biopsy. Am J Surg. 2004. 187:679–683.
Article8. Wahl RL, Cody RL, Hutchins GD, Mudgett EE. Primary and metastatic breast carcinoma: Initial clinical evaluation with PET with the radiolabeled glucose analogue 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1991. 179:765–770.
Article9. Adler LP, Crowe JP, al-Kaisi NK, Sunshine JL. Evaluation of breast masses and axillary lymph nodes with [F-18] 2-deoxy-2-fluoro-D-glucose PET. Radiology. 1993. 187:743–750.
Article10. Utech CI, Young CS, Winter PF. Prospective evaluation of fluorine-18 fluorodeoxyglucose positron emission tomography in breast cancer for staging of the axilla related to surgery and immunocytochemistry. Eur J Nucl Med. 1996. 23:1588–1593.
Article11. Avril N, Dose J, Jänicke F, Zieqler S, Römer W, Weber W. Assessment of axillary lymph node involvement in breast cancer patients with positron emission tomography using radiolabeled 2-(fluorine-18)-fluoro-2-deoxy-D-glucose. J Natl Cancer Inst. 1996. 88:1204–1209.
Article12. Adler LP, Faulhaber PF, Schnur KC, Al-Kasi NL, Shenk RR. Axillary lymph node metastases: screening with [F-18]2-deoxy-2-fluoro-D-glucose (FDG) PET. Radiology. 1997. 203:323–327.
Article13. Tatsumi M, Cohade C, Mourtzikos KA, Fishman EK, Wahl RL. Initial experience with FDG-PET/CT in the evaluation of breast cancer. Eur J Nucl Med Mol Imaging. 2006. 33:254–262.
Article14. de Kanter AY, van Eijck CH, van Geel AN, Kruijt RH, Henzen SC, Paul MA. Multicentre study of ultrasonographically guided axillary node biopsy in patients with breast cancer. Br J Surg. 1999. 86:1459–1462.
Article15. Pamilo M, Soiva M, Lavast EM. Real-time ultrasound, axillary mammography, and clinical examination in the detection of axillary lymph node metastases in breast cancer patients. J Ultrasound Med. 1989. 8:115–120.
Article16. Mustonen P, Farin P, Kosunen O. Ultrasonographic detection of metastatic axillary lymph nodes in breast cancer. Ann Chir Gynaecol. 1990. 79:15–18.17. Noh DY, Yun IJ, Kang HS, Kim JS, Lee DS, Chung JK, et al. The diagnostic value of positron emission tomography in detecting the breast cancer. J Korean Breast Cancer Soc. 1998. 1:6–12.
Article18. Barranger E, Grahek D, Antoine M, Montravers F, Talbot JN, Uzan S. Evaluation of fluorodeoxyglucose positron emission tomography in the detection of axillary lymph node metastasis in patients with early stage breast cancer. Ann Surg Oncol. 2003. 10:622–627.
Article19. Veronesi U, De Cicco C, Galimberti VE, Fernandez JR, Rotmensz N, Viale G, et al. A comparative study in the value of FDG-PET and sentinel node biopsy to identify occult axillary metastases. Ann Oncol. 2007. 18:473–478.
Article20. Taira N, Ohsumi S, Takabatake D, Hara F, Takashima S, Aogi K, et al. Determination of indication for sentinel lymph node biopsy in clinical node-negative breast cancer using preoperative 18F-fluoro-deoxyglucose positron emission tomography/computed tomography fusion imaging. Jpn J Clin Oncol. 2009. 39:16–21.
Article21. Ueda S, Tsuda H, Asakawa H, Omata J, Fukatsu K, Kondo N, et al. Utility of 18F-fluoro-deoxyglucose emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer. 2008. 8:165.
Article22. Wahl RL, Siegel BA, Coleman RE, Gatsonis CG. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004. 22:277–285.
Article23. Fehr MK, Hornung R, Varga Z, Burger D, Hess T, Haller U, et al. Axillary staging using positron emission tomography in breast cancer patients qualifying for sentinel lymph node biopsy. Breast J. 2004. 10:89–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Preoperative 18F-FDG PET/CT in Breast Cancer Patients in Comparison to the Conventional Imaging Study
- 18F-FDG PET/CT Findings in a Breast Cancer Patient with Concomitant Tuberculous Axillary Lymphadenitis
- F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer
- Hypermetabolic Axillary Mass on 18F FDG PET/CT: Breast Cancer Arising from Accessory Breast Tissue
- Use of 18F-FDG PET/CT in Second Primary Cancer