J Breast Cancer.
2008 Jun;11(2):71-76. 10.4048/jbc.2008.11.2.71.
Predictive Factors and Survival Rate for Brain Metastasis from Breast Cancer
- Affiliations
-
- 1Department of Surgery, Korea University College of Medicine, Seoul, Korea. kujwbae@korea.ac.kr
- KMID: 2241934
- DOI: http://doi.org/10.4048/jbc.2008.11.2.71
Abstract
- PURPOSE
The incidence of symptomatic brain metastases from breast cancerhas ranged from 10% to 16%. Brain metastases are traditionally viewed as a late complication of systemic disease, for which few effective treatment options exist. The aim of this study was to evaluate the factors that can predict brain metastases and to analyze the survival rate as compared with other systemic metastases.
METHODS
Between February 1983 and October 2005, 119 patient who developed systemic metastasis during the follow up period after optimal surgical treatment at Korea University Hospital were included in this study. Twenty-nine of these 119 patients had complaints of symptoms and they were consequently diagnosed as having brain metastases.
RESULTS
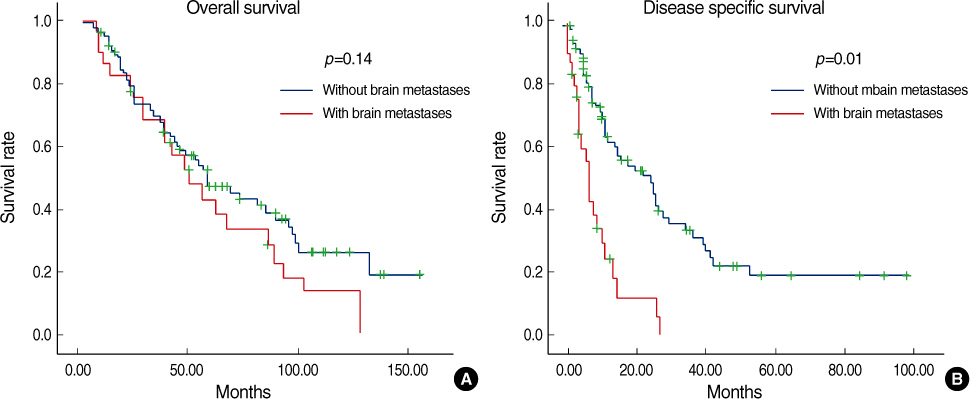
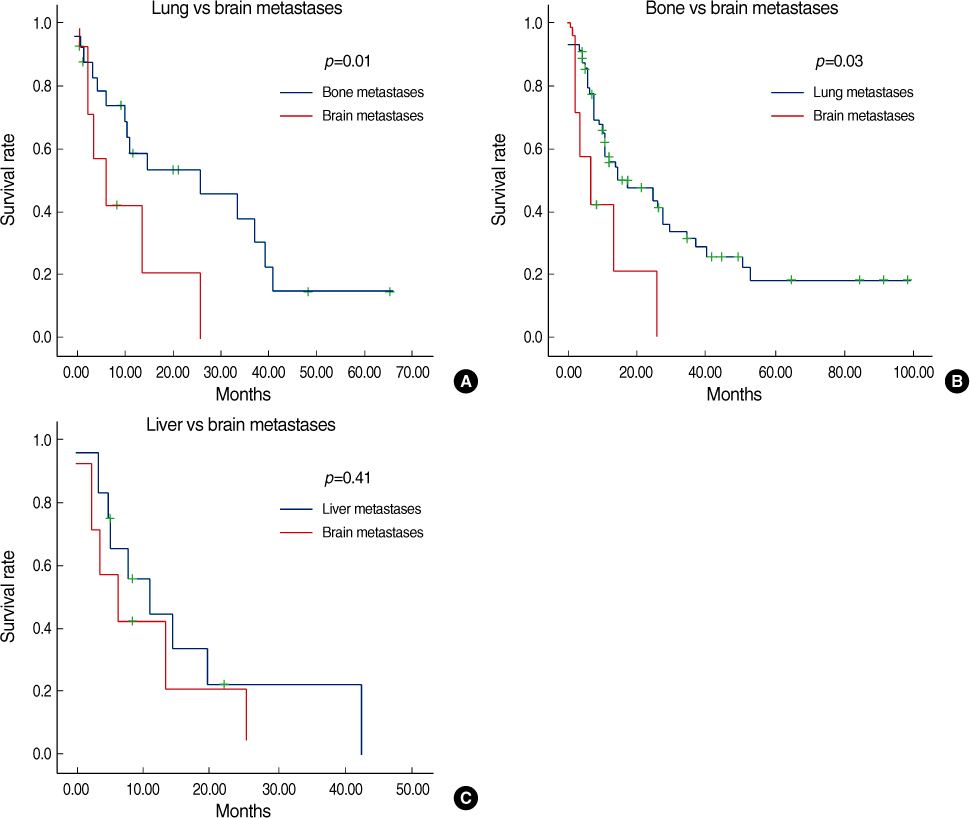
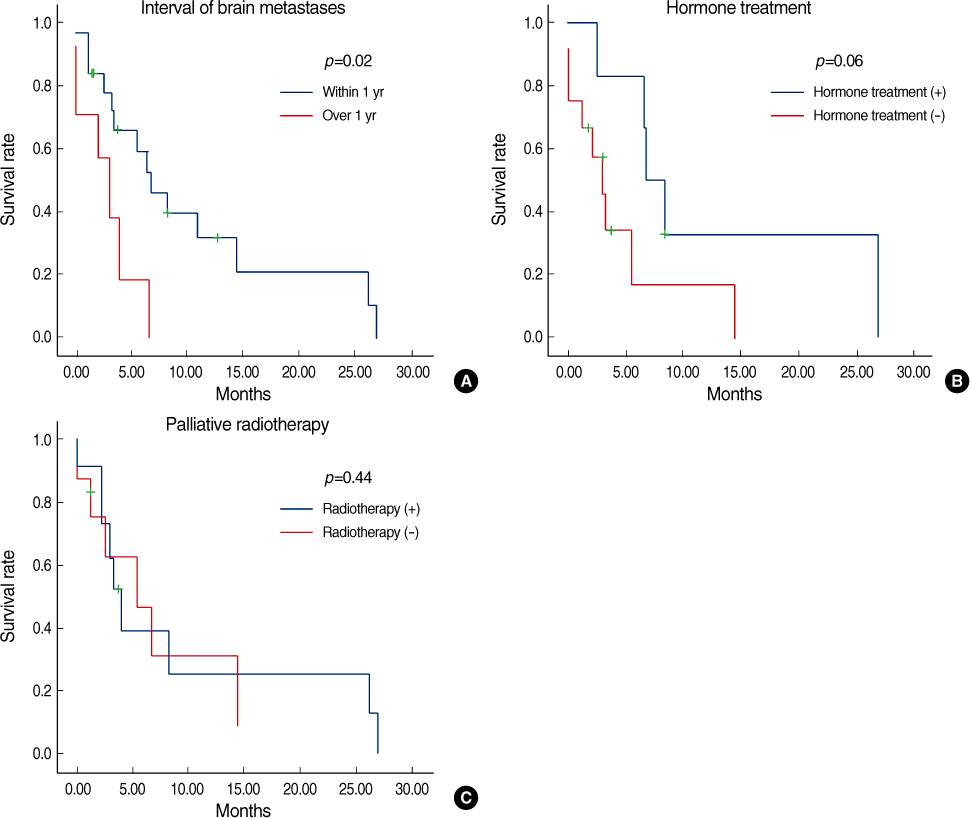
Estrogen receptor (-), progesterone receptor (-) and Her2 (-), and Triple negative were a significantly higher in the patients with brain metastases (p=0.04). The other clinicopathologic factors showed no difference between the patients with brain metastases and the patients without brain metastases. Lung metastases existed previously more often than the other systemic metastases (p=0.04). The overall survival of the patients with brain metastases was not significantly different from the patients with other systemic metastases. However, the disease specific survival of patients with brain metastases, as compared with other systemic metastases was poorer than that for patients with bone and lung metastases, respectively (p=0.01 and 0.03). A poor prognosis was shown for the cases with brain metastases within 1 yr after operation.
CONCLUSION
Clinician should give attention to the possibility of brain metastases for the breast cancer patients with triple negative findings or the patients who have developed lung metastases as this represents a symptom of central nervous system.
Keyword
MeSH Terms
Figure
Reference
-
1. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004. 22:3608–3617.
Article2. Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003. 21:1–23.
Article3. Chang EL, Lo S. Diagnosis and management of central nervous system metastases from breast cancer. Oncologist. 2003. 8:398–410.
Article4. Evans AJ, James JJ, Cornford EJ, Chan SY, Burrell HC, Pinder SE, et al. Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol. 2004. 16:345–349.
Article5. Issa CM, Semrau R, Kath R, Hoffken K. Isolated brain metastases as the sole manifestation of a late relapse in breast cancer. J Cancer Res Clin Oncol. 2002. 128:61–63.
Article6. Engel J, Eckel R, Aydemir U, Aydemir S, Kerr J, Schlesinger-Raab A, et al. Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys. 2003. 55:1186–1195.
Article7. Shaffrey ME, Mut M, Asher AL, Burri SH, Chahlavi A, Chang SM, et al. Brain metastases. Curr Probl Surg. 2004. 41:665–741.
Article8. Clark GM, Sledge GW Jr, Osborne CK, McGuire WL. Survival from first recurrence: relative importance of prognostic factors in 1,015 breast cancer patients. J Clin Oncol. 1987. 5:55–61.
Article9. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987. 235:177–182.
Article10. Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003. 97:2972–2977.
Article11. Lai R, Dang CT, Malkin MG, Abrey LE. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004. 101:810–816.
Article12. Miller KD, Weathers T, Haney LG, Timmerman R, Dickler M, Shen J, et al. Occult central nervous system involvement in patients with metastatic breast cancer: prevalence, predictive factors and impact on overall survival. Ann Oncol. 2003. 14:1072–1077.
Article13. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997. 37:745–751.
Article14. Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999. 43:795–803.
Article15. Shmueli E, Wigler N, Inbar M. Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer. 2004. 40:379–382.
Article16. Kallioniemi OP, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer. 1991. 49:650–655.
Article17. Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004. 91:639–643.
Article18. Tsuda H, Takarabe T, Hasegawa F, Fukutomi T, Hirohashi S. Large, central acellular zones indicating myoepithelial tumor differentiation in high-grade invasive ductal carcinomas as markers of predisposition to lung and brain metastases. Am J Surg Pathol. 2000. 24:197–202.
Article19. Rodriguez-Pinilla SM, Sarrio D, Honrado E, Hardisson D, Calero F, Benitez J, et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006. 12:1533–1539.
Article20. Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004. 350:1655–1664.
Article21. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002. 2:584–593.
Article22. Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery. Ann Neurol. 1993. 33:583–590.
Article23. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990. 322:494–500.
Article24. Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996. 78:1470–1476.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Features and Prognosis of Lung Cancer with Brain Metastasis
- Incidence of Brain Metastasis and Related Subtypes in Patients with Breast Cancer Receiving Adjuvant Radiation Therapy after Surgery
- Familial Breast Cancer
- Clinical Outcomes in Patients with Triple-negative Breast Cancer and Brain Metastases
- Advances in Clinically Relevant Metastatic Breast Cancer Models