Korean J Gastroenterol.
2014 Jun;63(6):335-340. 10.4166/kjg.2014.63.6.335.
Clinical Significance of Hepatitis B Surface Antigen Quantification in Chronic Hepatitis B
- Affiliations
-
- 1Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. jeyyeon@hotmail.com
- KMID: 2234033
- DOI: http://doi.org/10.4166/kjg.2014.63.6.335
Abstract
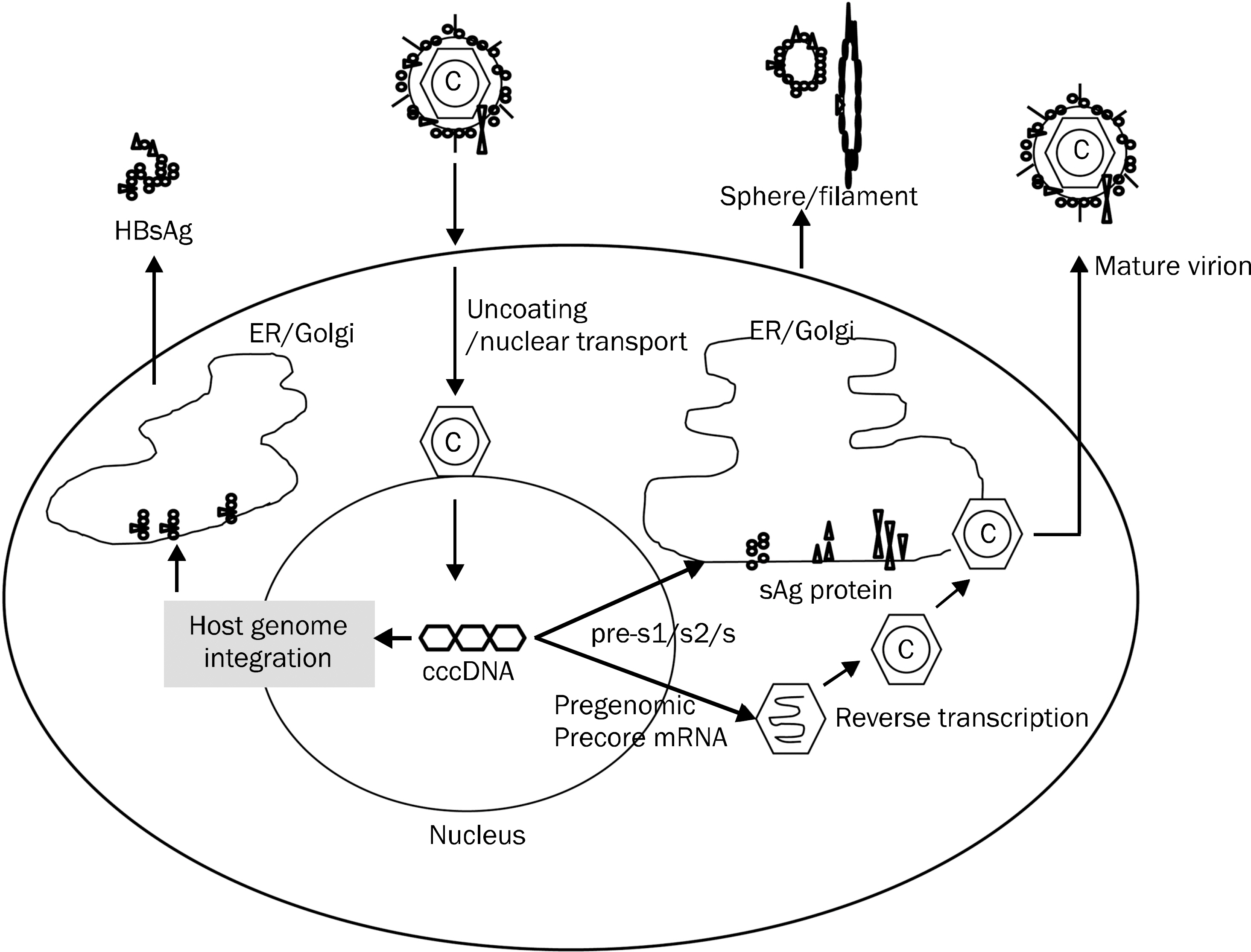
- Since the discovery of HBsAg in the early 1960s, presence of HBsAg in serum has only served to diagnose hepatitis B. Recent development in the quantitative measurement of serum HBsAg has enabled us to improve our understanding on the management of chronic hepatitis B. The surface antigen (sAg) level is at its highest in immune tolerance phase and decreases to the lowest level in immune control/inactive phase when HBeAg is cleared from the serum. Combination of serum sAg titer less than 1,000 IU/mL and serum HBV DNA less than 2,000 IU/mL can identify true inactive carrier from e antigen (eAg) negative hepatitis with diagnostic accuracy of 95%. During the natural course of chronic hepatitis B, changes or absolute level of sAg less than certain level can predict spontaneous sero-clearance of HBsAg. Although the decline of sAg is very slow in interferon (IFN)/pegylated interferon (PEG-IFN) or oral nucleos(-t)ide treated patients, interferon based therapy results in a greater decrease of sAg level and sAg loss. Lack of any decline in sAg titer during PEG-IFN therapy could identify the group of patients who do not response to IFN/PEG-IFN therapy. With the aid of serum HBV DNA, quantitative measurement of serum HBsAg level can be used to optimize the management of chronic hepatitis B in our daily practice.
MeSH Terms
-
Antiviral Agents/therapeutic use
DNA, Viral/blood
Hepatitis B Surface Antigens/*blood
Hepatitis B e Antigens/blood
Hepatitis B, Chronic/*diagnosis/drug therapy/genetics
Humans
Interferons/therapeutic use
Liver Neoplasms/diagnosis
Prognosis
Antiviral Agents
DNA, Viral
Hepatitis B Surface Antigens
Hepatitis B e Antigens
Interferons
Figure
Reference
-
References
1. Chen CJ, Yang HI, Su J, et al. REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006; 295:65–73.
Article2. Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004; 126:1750–1758.3. Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: a review. Hepatology. 2011; 54:E1–E9.
Article4. Hadziyannis E, Hadziyannis SJ. Hepatitis B surface antigen quantification in chronic hepatitis B and its clinical utility. Expert Rev Gastroenterol Hepatol. 2014; 8:185–195.
Article5. Tuaillon E, Mondain AM, Nagot N, et al. Comparison of serum HBsAg quantitation by four immunoassays, and relationships of HBsAg level with HBV replication and HBV genotypes. PLoS One. 2012; 7:e32143.
Article6. Nguyen T, Thompson AJ, Bowden S, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010; 52:508–513.
Article7. Tong MJ, Nguyen MO, Tong LT, Blatt LM. Development of hepatocellular carcinoma after seroclearance of hepatitis B surface antigen. Clin Gastroenterol Hepatol. 2009; 7:889–893.
Article8. Chu CM, Liaw YF. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir Ther. 2010; 15:133–143.
Article9. Yuen MF, Wong DK, Fung J, et al. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008; 135:1192–1199.
Article10. Lauret E, González-Diéguez ML, Rodríguez M, et al. Longterm outcome in Caucasian patients with chronic hepatitis B virus infection after HBsAg seroclearance. Liver Int. 2014. DOI: doi: 10.1111/liv.12461.
Article11. Tseng TC, Liu CJ, Yang HC, et al. Determinants of spontaneous surface antigen loss in hepatitis B e antigen-negative patients with a low viral load. Hepatology. 2012; 55:68–76.
Article12. Chan HL, Wong VW, Wong GL, Tse CH, Chan HY, Sung JJ. A longitudinal study on the natural history of serum hepatitis B surface antigen changes in chronic hepatitis B. Hepatology. 2010; 52:1232–1241.
Article13. Brunetto MR, Oliveri F, Colombatto P, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology. 2010; 139:483–490.
Article14. Tseng TC, Liu CJ, Yang HC, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012; 142:1140–1149. e3; quiz e13–14.
Article15. Brunetto MR, Moriconi F, Bonino F, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009; 49:1141–1150.
Article16. Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky JM. Longterm hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013; 58:676–683.
Article17. Reijnders JG, Rijckborst V, Sonneveld MJ, et al. Kinetics of hepatitis B surface antigen differ between treatment with peginterferon and entecavir. J Hepatol. 2011; 54:449–454.
Article18. Seto WK, Lam YF, Fung J, et al. Changes of HBsAg and HBV DNA levels in Chinese chronic hepatitis B patients after 5 years of entecavir treatment. J Gastroenterol Hepatol. 2014; 29:1028–1034.
Article19. Sonneveld MJ, Rijckborst V, Boucher CA, Hansen BE, Janssen HL. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology. 2010; 52:1251–1257.
Article20. Chan HL, Wong VW, Chim AM, Chan HY, Wong GL, Sung JJ. Serum HBsAg quantification to predict response to peginterferon therapy of e antigen positive chronic hepatitis B. Aliment Pharmacol Ther. 2010; 32:1323–1331.
Article21. Ma H, Yang RF, Wei L. Quantitative serum HBsAg and HBeAg are strong predictors of sustained HBeAg seroconversion to pegylated interferon alfa-2b in HBeAg-positive patients. J Gastroenterol Hepatol. 2010; 25:1498–1506.
Article22. Moucari R, Mackiewicz V, Lada O, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009; 49:1151–1157.
Article23. Rijckborst V, Hansen BE, Ferenci P, et al. Validation of a stopping rule at week 12 using HBsAg and HBV DNA for HBeAg-negative patients treated with peginterferon alfa-2a. J Hepatol. 2012; 56:1006–1011.
Article24. Jung YK, Kim JH, Lee YS, et al. Change in serum hepatitis B surface antigen level and its clinical significance in treatmentnaïve, hepatitis B e antigen-positive patients receiving entecavir. J Clin Gastroenterol. 2010; 44:653–657.
Article25. Wursthorn K, Jung M, Riva A, et al. Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology. 2010; 52:1611–1620.
Article26. Cai W, Xie Q, An B, et al. On-treatment serum HBsAg level is predictive of sustained off-treatment virologic response to telbivudine in HBeAg-positive chronic hepatitis B patients. J Clin Virol. 2010; 48:22–26.
Article27. Lee JM, Ahn SH, Kim HS, et al. Quantitative hepatitis B surface antigen and hepatitis B e antigen titers in prediction of treatment response to entecavir. Hepatology. 2011; 53:1486–1493.
Article28. Seto WK, Liu K, Wong DK, et al. Patterns of hepatitis B surface antigen decline and HBV DNA suppression in Asian treatment-experienced chronic hepatitis B patients after three years of tenofovir treatment. J Hepatol. 2013; 59:709–716.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Growing attention to an old marker, hepatitis B surface antigen, in the natural history of chronic hepatitis B
- Quantitative hepatitis B surface antigen predicts the antiviral response and hepatocellular carcinoma development in patients with chronic hepatitis B
- Prevalence of hepatitis B surface antigen in pediatric in patients
- A Study on Periphral T Cell Subsets in Asymptomatic HBsAg Carriers and Children with Chronic Hepatitis B and Hepatitis B vaccine Inoculated Infants
- The Eligibility Study of Anesthesia and Surgery for HBs Antigen Positive Patients