Korean Circ J.
2007 Oct;37(10):503-509. 10.4070/kcj.2007.37.10.503.
Postoperative Outcomes of Patients with Severe Aortic Regurgitation and Decreased Left Ventricular Ejection Fraction
- Affiliations
-
- 1Division of Cardiology, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, Korea. jksong@amc.seoul.kr
- 2Division of Thoracic and Cardiovascular Surgery, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, Korea.
- KMID: 2227052
- DOI: http://doi.org/10.4070/kcj.2007.37.10.503
Abstract
-
BACKGROUND AND OBJECTIVES: The postoperative outcomes of patients with severe aortic regurgitation (AR) and a low ejection fraction (EF) were elucidated.
SUBJECTS AND METHODS
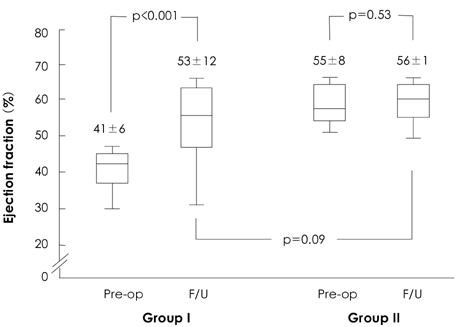
The study group consisted of 201 consecutive patients that underwent corrective surgery for isolated AR. The clinical data of patients with a preoperative EF<50% (n=59, group I) and of patients with an EF> or =50% (n=142, group II) were compared. The clinical follow-up duration was 3.2+/-2.4 years.
RESULTS
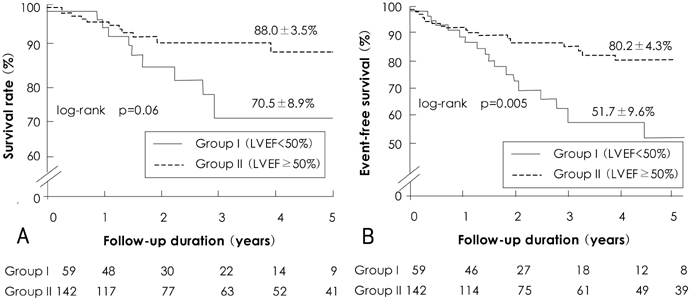
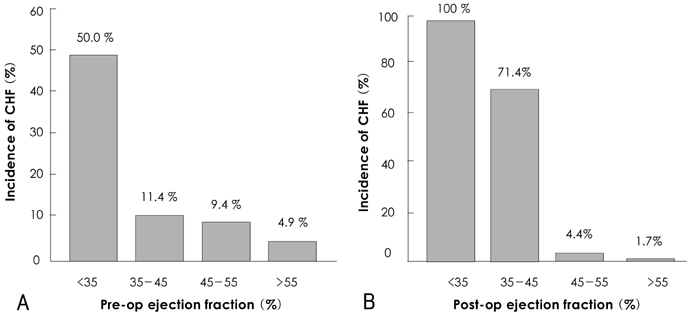
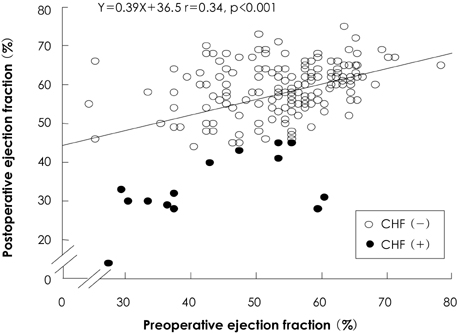
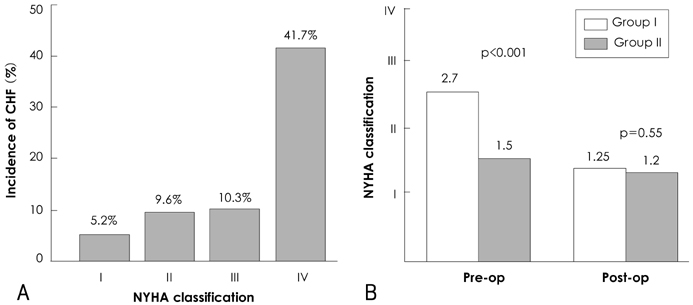
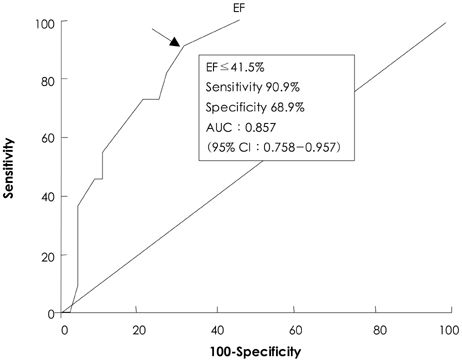
There was no operative mortality for patients in both groups. Group I patients showed significant improvement of the EF after surgery, from 41+/-6% to 53+/-12% (p<0.001) and the EF (53+/-12 vs 56+/-1%, p=0.09) at follow-up was not significantly different between patients in the two groups. The 5-year survival rate was 70.5+/-8.9% for group I patients and 88.0+/-3.5% for group II patients (p=0.06). The cumulative incidence of congestive heart failure at 5 years was significantly higher in group I patients (32.1+/-9.7% vs 8.5+/-3.1%, p=0.003). Independent determinants of development of congestive heart failure in group I patients were age [hazards ratio (HR)=1.1, 95% confidence interval (CI)=1.02-1.16, p=0.01] and EF (HR=0.82, 95% CI=0.69-0.97, p=0.02). The best cut-off value of the preoperative EF in predicting the development of congestive heart failure was 41.5%, with a sensitivity and specificity of 90.9% and 68.9%, respectively.
CONCLUSION
A relatively high 5-year survival rate without operative mortality is anticipated in patients with reduced a preoperative left ventricular ejection fraction (LVEF) and the incidence of congestive heart failure was higher when compared to patients with a normal preoperative LVEF, which could be predicted by the level of the preoperative LVEF.
Keyword
MeSH Terms
Figure
Reference
-
1. Tarasoutchi F, Grinberg M, Spina GS, et al. Ten-year clinical laboratory follow-up after application of a symptom-based therapeutic strategy to patients with severe chronic aortic regurgitation of predominant rheumatic etiology. J Am Coll Cardiol. 2003. 41:1316–1324.2. Bonow RO. Radionuclide angiography in the management of asymptomatic aortic regurgitation. Circulation. 1991. 84:Suppl. I296–I302.3. Dujardin KS, Enriquez-Sarano M, Schaff HV, Bailey KR, Seward JB, Tajik AJ. Mortality and morbidity of aortic regurgitation in clinical practice: a long-term follow-up study. Circulation. 1999. 99:1851–1857.4. Bonow RO, Carabello B, Chatterjee K, et al. ACC/AHA guidelines for the treatment of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). J Am Coll Cardiol. 2006. 48:e1-148.5. Borer JS, Hochreiter C, Herrold EM, et al. Prediction of indications for valve replacement among asymptomatic or minimally symptomatic patients with chronic aortic regurgitation and normal left ventricular performance. Circulation. 1998. 97:525–534.6. Bonow RO, Lakatos E, Maron BJ, Epstein SE. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation. 1991. 84:1625–1635.7. Aronow WS, Ahn C, Kronzon I, Nanna M. Prognosis of patients with heart failure and unoperated severe aortic valvular regurgitation and relation to ejection fraction. Am J Cardiol. 1994. 74:286–288.8. Chaliki HP, Mohty D, Avierinos JF, et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation. 2002. 106:2687–2693.9. Kim HS, Song JK, Lee JH, et al. Short and long-term results of open heart surgery in aortic valve disease. Korean Circ J. 1998. 28:1509–1517.10. Perry GJ, Helmcke F, Nanda NC, Byard C, Soto B. Evaluation of aortic insufficiency by Doppler color flow mapping. J Am Coll Cardiol. 1987. 9:952–959.11. Teague SM, Heinsimer JA, Anderson JL, et al. Quantification of aortic regurgitation utilizing continuous wave Doppler ultrasound. J Am Coll Cardiol. 1986. 8:592–599.12. Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981. 64:744–753.13. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989. 2:358–367.14. Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001. 37:885–892.15. Bonow RO, Picone AL, McIntosh CL, et al. Survival and functional results after valve replacement for aortic regurgitation from 1976 to 1983: impact of preoperative left ventricular function. Circulation. 1985. 72:1244–1256.16. Henry WL, Bonow RO, Borer JS, et al. Observations on the optimum time for operative intervention for aortic regurgitation: I. evaluation of the results of aortic valve replacement in symptomatic patients. Circulation. 1980. 61:471–483.17. Klodas E, Enriquez-Sarano M, Tajik AJ, Mullany CJ, Bailey KR, Seward JB. Aortic regurgitation complicated by extreme left ventricular dilation: long-term outcome after surgical correction. J Am Coll Cardiol. 1996. 27:670–677.18. Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002. 346:1128–1137.19. Chung IM, Cho SY, Park SJ, et al. An echocardiographic study of left ventricular functional change in pure aortic regurgitation patients after aortic valve replacement. Korean Circ J. 1987. 17:661–672.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Left ventricular function after mitral valve operation in congenital mitral regurgitation
- Comparison of Postoperative LV Function after Mitral Valve Replacement and Predictor of Postoperative LV Function in Chronic Mitral Regurgitation
- Regression of Left Ventricular Mass after Aortic Valve Replacement in Isolated Aortic Regurgitation
- Exercise Echocardiography in Patients with Chronic Aortic Regurgitation: A Serial Echocardiographic and Clinical Follow-up Study
- Evaluation of Ejection Fraction Obtained by Echocardiography and Radionuclide Ventriculography