Korean Circ J.
2009 Sep;39(9):359-366. 10.4070/kcj.2009.39.9.359.
Cardioprotective Effects of Alpha-Lipoic Acid on Myocardial Reperfusion Injury: Suppression of Reactive Oxygen Species Generation and Activation of Mitogen-Activated Protein Kinase
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea. oskcar@wonkwang.ac.kr
- 2Department of Physiology, Wonkwang University School of Medicine, Iksan, Korea.
- KMID: 2225671
- DOI: http://doi.org/10.4070/kcj.2009.39.9.359
Abstract
- BACKGROUND AND OBJECTIVES
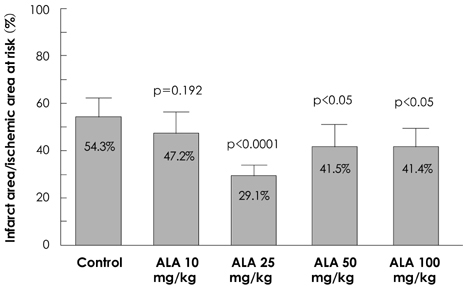
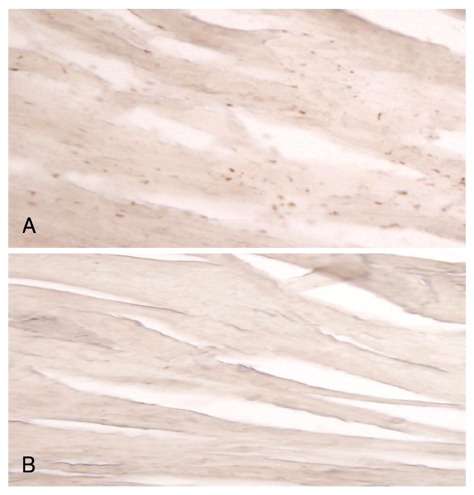
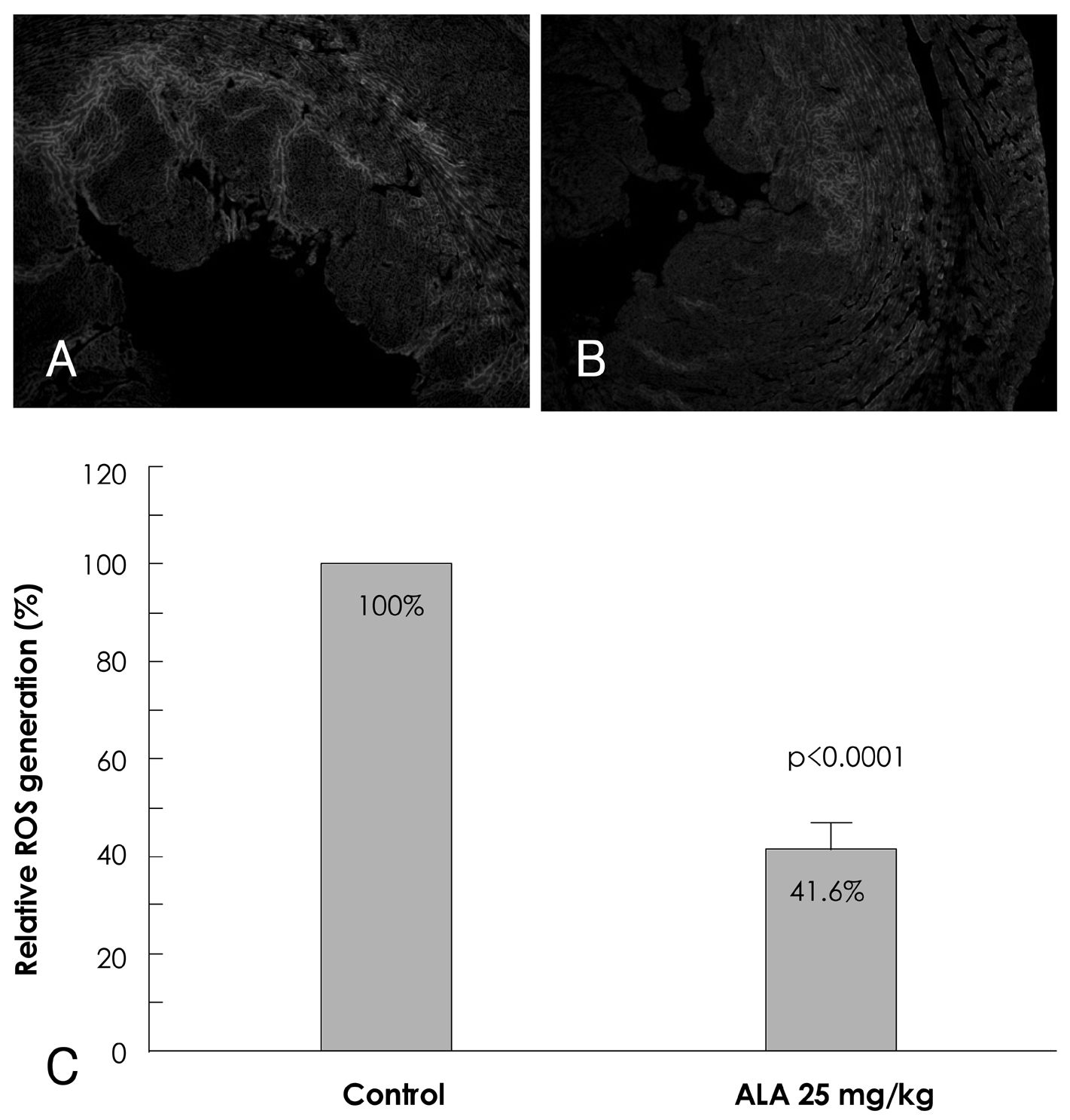
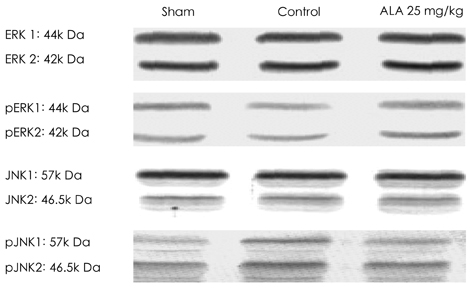
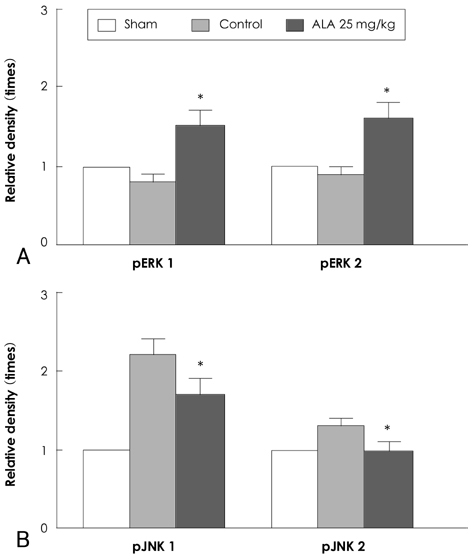
Reactive oxygen species (ROS) and mitogen-activated protein (MAP) kinase play an important role in the development of myocardial reperfusion injury. In this study, we examined whether treatment with alpha-lipoic acid (ALA) before reperfusion could prevent myocardial reperfusion injury in vivo. Materials and Methods: Sprague-Dawley rats were subjected to a 45-minute left anterior descending coronary artery ligation followed by 45- or 10-minute reperfusion. ALA was administered 10 minutes prior to reperfusion. The infarct size ratio of the infarct area to the ischemic area at risk, was measured based on 10, 25, 50, and 100 mg/kg of ALA, with propidium iodide (PI) fluorescence. Apoptosis was evaluated by TdT-mediated dUDP nick end labeling (TUNEL) staining. The generation of intracellular ROS was evaluated using the fluorogenic probe, dichlorodihydrofluorescein diacetate (CM-H2DCFDA). Western blot analysis was performed for MAP kinase (pERK 1/2 and pJNK 1/2) activity. RESULTS: The infarct size, according to ALA dose, was significantly suppressed 29.1% with ALA 25 mg/kg (p<0.0001), 41.5% with 50 mg/kg (p<0.05), and 41.4% with 100 mg/kg (p<0.05) compared to the controls (54.3%). However, the results were not significantly different with 47.2% of the ALA 10 mg/kg (p=0.192). A few apoptotic nucleoli were detected in the ALA 25 mg/kg group, but were frequently detected in the control group. The ROS generation was significantly suppressed (p<0.0001), the activity of pERK 1/2 was significantly increased (p<0.05) and the activity of pJNK 1/2 was significantly decreased (p<0.05) in the ALA 25 mg/kg group compared to the controls. CONCLUSION: The results of this study suggested that adequate doses of ALA before reperfusion was effective for the prevention of myocardial reperfusion injury in vivo. This cardioprotective activity of ALA might be associated with an anti-apoptotic effect of ALA via suppression of ROS generation, increase of pERK 1/2 and decrease of pJNK 1/2 activity.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Coronary Vessels
Fluorescence
Ligation
Myocardial Reperfusion
Myocardial Reperfusion Injury
Phosphotransferases
Propidium
Protein Kinases
Rats, Sprague-Dawley
Reactive Oxygen Species
Reperfusion
Thioctic Acid
Phosphotransferases
Propidium
Protein Kinases
Reactive Oxygen Species
Thioctic Acid
Figure
Reference
-
1. Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985. 76:1713–1719.2. Eefting F, Rensing B, Wigman J, et al. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004. 61:414–426.3. Zhao ZQ, Nakamura M, Wang NP, et al. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res. 2000. 45:651–660.4. Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998. 93:Suppl 3. 8–12.5. Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart: evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988. 263:1353–1357.6. Cicconi S, Ventura N, Pastore D, et al. Characterization of apoptosis signal transduction pathways in HL-5 cardiomyocytes exposed to ischemia/reperfusion oxidative stress model. J Cell Physiol. 2003. 195:27–37.7. Abe J, Baines CP, Berk BC. Role of mitogen-activated protein kinases in ischemia and reperfusion injury: the good and the bad. Circ Res. 2000. 86:607–609.8. Omura T, Yoshiyama M, Shimada T, et al. Activation of mitogen-activated protein kinases in in vivo ischemia/reperfused myocardium in rats. J Mol Cell Cardiol. 1999. 31:1269–1279.9. Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc Res. 2000. 47:446–456.10. Marczin N, El-Habashi N, Hoare GS, Bundy RE, Yacoub M. Antioxidants in myocardial ischemia-reperfusion injury: therapeutic potential and basic mechanisms. Arch Biochem Biophys. 2003. 420:222–236.11. Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997. 29:315–331.12. Freisleben HJ. Lipoic acid reduces ischemia-reperfusion injury in animal models. Toxicology. 2000. 148:159–171.13. Schonheit K, Gille L, Nohl H. Effect of alpha-lipoic acid and dihydrolipoic acid on ischemia/reperfusion injury of the heart and heart mitochondria. Biochem Biophys Acta. 1995. 1271:335–342.14. Cao Z, Tsang M, Zhao H, Li Y. Induction of endogenous anti-oxidants and phase 2 enzymes by alpha-lipoic acid in rat cardiac H9C2 cells: protection against oxidative injury. Biochem Biophys Res Commun. 2003. 310:979–985.15. Wolff RA, Chien GL, van Winkle DM. Propidium iodide compares favorably with histology and triphenyl tetrazolium chloride in the assessment of experimentally-induced infarct size. J Mol Cell Cardiol. 2000. 32:225–232.16. Doi K, Suzuki Y, Nakao A, Fujita T, Noiri E. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int. 2004. 65:1714–1723.17. Kim H, Park CK, Kim KS. Antioxidants inhibit smooth muscle cell proliferation in vitro and neointimal hyperplasia in vivo after carotid artery injury in the rat. Korean Circ J. 2004. 34:69–75.18. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.19. Kajstura J, Chen W, Reiss K, et al. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996. 74:86–107.20. Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996. 79:949–956.21. Ambrosio G, Zweier JL, Flaherty JT. The relationship between oxygen radical generation and impairment of myocardial energy metabolism following post-ischemic reperfusion. J Mol Cell Cardiol. 1991. 23:1359–1374.22. Duilio C, Ambrosio G, Kuppusamy P, Dipaula A, Becker LC, Zweier JL. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001. 280:H2649–H2657.23. Bolli R, Jeroudi MO, Patel BS, et al. Marked reduction of free radial generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion: evidence that myocardial stunning is a manifestation of reperfusion injury. Circ Res. 1989. 65:607–622.24. Kim TY, Chung HM, Park HJ, et al. The cardioprotective effects of resveratrol via anti-apoptosis in hypoxic injury of myocardial cells. Korean Circ J. 2007. 37:408–413.25. Koh JH, Ryu KH, Lim SH, Hong KS, Choi YJ, Park SW. Effect of antioxidants on myocardial damage in streptozotocin-induced diabetic rats. Korean Circ J. 2006. 36:261–271.26. Scarabelli T, Stephanou A, Rayment N, et al. Apoptosis of endothelial cells precedes myocyte cell apoptosis in ischemia/reperfusion injury. Circulation. 2001. 104:253–256.27. Dun Y, Zhi JM, Sun HY, Zhao RR, Zhao ZQ. Activated polymorphonuclear leukocytes induce cardiomyocyte apoptosis and the protective effects of carvedilol. Methods Find Exp Clin Pharmacol. 2002. 24:403–412.28. Zhao ZQ. Oxidative stress-elicited myocardial apoptosis during reperfusion. Curr Opin Pharmocol. 2004. 4:159–165.29. Yue TL, Wang C, Gu JL, et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000. 86:692–699.30. Ferrandi C, Ballerio R, Gaillard P, et al. Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte apoptosis and infarct size after myocardial ischemia and reperfusion in anaesthetized rats. Br J Pharmacol. 2004. 142:953–960.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- alpha-Lipoic Acid Inhibits Expression of IL-8 by Suppressing Activation of MAPK, Jak/Stat, and NF-kappaB in H. pylori-Infected Gastric Epithelial AGS Cells
- Mechanism of Ischemia and Reperfusion Injury to the Heart: From the Viewpoint of Nitric Oxide and Mitochondria
- Molecular Mechanisms of Neutrophil Activation in Acute Lung Injury
- The Effects of Glucocorticoid and alpha-Lipoic Acid on Ischemia-Reperfusion Injury
- Molecular Mechanism of Reactive Oxygen Species-dependent ASK1 Activation in Innate Immunity