Korean Circ J.
2012 Mar;42(3):154-163. 10.4070/kcj.2012.42.3.154.
A Comparative Study on Mechanical and Biochemical Properties of Bovine Pericardium After Single or Double Crosslinking Treatment
- Affiliations
-
- 1Seoul National University Hospital, Clinical Research Institute, Xenotransplantation Research Center, Seoul, Korea. kyj@plaza.snu.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, Korea.
- KMID: 2225029
- DOI: http://doi.org/10.4070/kcj.2012.42.3.154
Abstract
- BACKGROUND AND OBJECTIVES
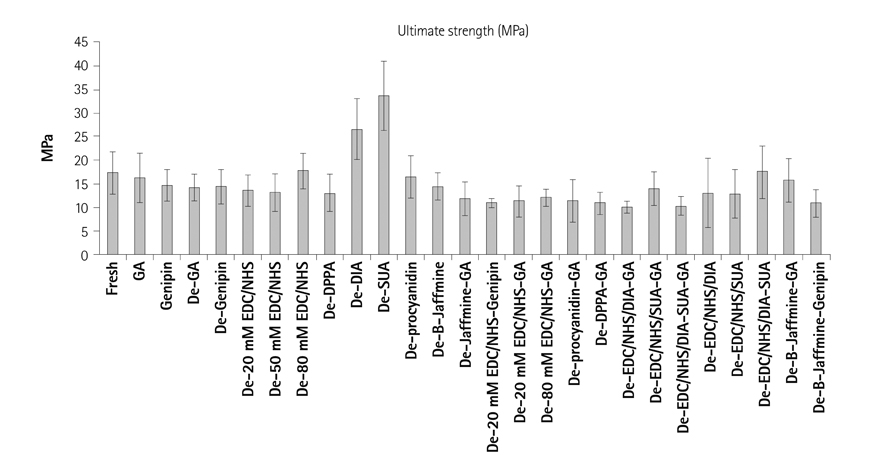
Glutaraldehyde (GA) has been used as a representative method of tissue preservation in cardiovascular surgery. However, GA has showed limited durability including calcification, mechanical failure and toxicity. To overcome those unsolved problems, we analyzed the crosslinking differences of primary amines, GA and genipin in their mechanical and biochemical properties with a single or double crosslinking agent for clinical application.
MATERIALS AND METHODS
Samples were divided into 3 groups; control, single crosslinking fixation and double crosslinking fixation after decellurarization using bovine pericardium. For analysis of the biochemical and mechanical properties of each crosslinking method, tensile strength, percentage strain, thermal stability, resistance to pronase, nynhydrin and cytotoxicity test were studied.
RESULTS
Combined hexamethylene diamine and suberic acid in the carbodiimide hydrochloride/N-hydroxysucinimide solution (EDC/NHS) after decellurarization, tensile strength and strain percentage were not statistically significant compared to the single crosslinking treated groups (p>0.05). Tissue crosslinking stability was weak in single treatment of diphenylphosphoryl azide, suberic acid, low concentration of EDC, hexamethylene diamine and procyanidin groups, but thermal stability and resistance to the pronase and ninhydrin were markedly increased in concentrated EDC/NHS or after combined double treatment with low concentration of GA or genipin (p<0.001).
CONCLUSION
Single or double crosslinking with low concentration of carbodiimide, diphenylphosphonyl azide, procyanidin, suberic acid and hexane diamine were not as effective in mechanical, biochemical, cytotoxic and crosslinking properties compared to GA or genipin fixation, but their mechanical and chemical properties were much improved when combined with low concentrations of GA or genipin in the double crosslinking process.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee CR, Grodzinsky AJ, Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials. 2001. 22:3145–3154.2. Speer DP, Chvapil M, Eskelton CD, Ulreich J. Biological effects of residual glutaraldehyde-tanned collagen biomaterials. J Biomed Mater Res. 1980. 14:753–764.3. Petite H, Rault I, Huc A, Menasche P, Herbage D. Use of the acyl azide method for cross-linking collagen-rich tissues such as pericardium. J Biomed Mater Res. 1990. 24:179–187.4. Anselme K, Petite H, Herbage D. Inhibition of calcification in vivo by acyl azide cross-linking of a collagen-glycosaminoglycan sponge. Matrix. 1992. 12:264–273.5. Petite H, Duval JL, Frei V, Abdul-Malak N, Sigot-Luizard MF, Herbage D. Cytocompatibility of calf pericardium treated by glutaraldehyde and by the acyl azide methods in an organotypic culture model. Biomaterials. 1995. 16:1003–1008.6. Olde Damink LH, Diijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996. 17:765–773.7. Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide. Biomaterials. 1996. 17:679–684.8. Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. Crosslinking of dermal sheep collagen using hexamethylene diisocyanate. J Mater Sci Mater Med. 1995. 6:429–434.9. Hanthamrongwit M, Reid WH, Grant MH. Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: the effect of cross-linking agents and diamines. Biomaterials. 1996. 17:775–780.10. Shen SH, Sung HW, Tu R, et al. Characterization of a polyepoxy compound fixed porcine heart valve bioprosthesis. J Appl Biomater. 1994. 5:159–162.11. Xi T, Ma J, Tian W, Lei X, Long S, Xi B. Prevention of tissue calcification on bioprosthetic heart valve by using epoxy compounds: a study of calcification test in vitro and in vivo. J Biomed Mater Res. 1992. 26:1241–1251.12. Nimni ME, Cheung DT, Strates B, Kodama M, Sheikh K. Nimni ME, editor. Bioprosthesis derived from cross-linked and chemically modified collagenous tissues. Collagen. 1988. Vol. III. Boca Raton, FL: CRC Press;1–38.13. Zeeman R, Dijkstra PJ, van Wachem PB, et al. Successive epoxy and carbodiimide cross-linking of dermal sheep collagen. Biomaterials. 1999. 20:921–931.14. Sung HW, Hsu CS, Wang SP, Hsu HL. Degradation potential of biological tissues fixed with various fixatives: an in vitro study. J Biomed Mater Res. 1997. 35:147–155.15. Timkovich R. Detection of the stable addition of carbodiimide to proteins. Anal Biochem. 1977. 79:135–143.16. Jang WS, Kim YJ, Kim SH. Effects on tensile strength and elasticity after treatment with glutaraldehyde, solvent, decellularization and detoxification in fresh bovine pericardium. Korean J Thorac Cardiovasc Surg. 2010. 43:1–10.17. Sung SC, Kim YJ, Choi SY, Park JE, Kim KW, Kim WH. A study on an effective decelularization technique for a xenograft cardiac valve: the effect of osmotic treatment with hypotonic solution. Korean J Thorac Cardiovasc Surg. 2008. 41:679–686.18. Cho S, Kim YJ, Kim SH, Choi SH. Biaxial strain analysis of various fixation models in porcine aortic and pulmonary valves. Korean J Thorac Cardiovasc Surg. 2009. 42:566–575.19. Cho S, Kim YJ, Kim SH, Park JE, Kim WH. Comparison of the uniaxial tensile strength, elasticity and thermal stability between glutaraldehyde and glutaraldehyde with solvent fixation in xenograft cardiovascular tissue. Korean J Thorac Cardiovasc Surg. 2009. 42:165.20. Hansen DB, Joullie MM. The development of novel ninhydrin analogues. Chem Soc Rev. 2005. 34:408–417.21. Jayakrishnan A, Jameela SR. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials. 1996. 17:471–484.22. Gratzer PF, Lee JM. Control of pH alters the type of cross-linking produced by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) treatment of acellular matrix vascular grafts. J Biomed Mater Res. 2001. 58:172–179.23. Moore MA, Chen WM, Phillips RE, Bohachevsky IK, McIlroy BK. Shrinkage temperature versus protein extraction as a measure of stabilization of photooxidized tissue. J Biomed Mater Res. 1996. 32:209–214.24. Zheng LT, Ryu GM, Kwon BM, Lee WH, Suk K. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: inhibition of microglial neurotoxicity. Eur J Pharmacol. 2008. 588:106–113.25. Dongmo AB, Kamanyi A, Anchang MS, et al. Anti-inflammatory and analgesic properties of the stem bark extracts of Erythrophleum suaveolens (Caesalpiniaceae), Guillemin & Perrottet. J Ethnopharmacol. 2001. 77:137–141.26. Blazsó G, Gábor M, Rohdewald P. Antiinflammatory activities of procyanidin-containing extracts from Pinus pinaster Ait: after oral and cutaneous application. Pharmazie. 1997. 52:380–382.27. Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic Biol Med. 1999. 27:704–724.28. Bretti C, Crea F, Foti C, Smmartano S. Solubility and activity coefficients of acidic and basic nonelectolytes in aqueous salt solutions. J Chem Eng Data. 2005. 50:1761–1767.29. Schmidt CE, Baier JM. Acellular vascular tissue: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000. 21:2215–2231.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bovine Pericardium as a Wrapping Material for Hydroxyapatite Orbital Implant
- Histologic Characteristics and Mechanical Properties of Bovine Pericardium Treated with Decellularization and alpha-Galactosidase: A Comparative Study
- Changes of the Structural and Biomechanical Properties of the Bovine Pericardium after the Removal of alpha-Gal Epitopes by Decellularization and alpha-Galactosidase Treatment
- Tracheal augmentation with Bovine pericardium
- The Dynamic and Histologic Changes of Variously Fixed Bovine Pericardiums Specimens after Mechanical Fatigue Stimuli