Korean Circ J.
2012 Nov;42(11):747-752. 10.4070/kcj.2012.42.11.747.
Positive Vascular Remodeling in Culprit Coronary Lesion is Associated With Plaque Composition: An Intravascular Ultrasound-Virtual Histology Study
- Affiliations
-
- 1Department of Cardiology, Konyang University College of Medicine, Daejeon, Korea. janghobae@yahoo.co.kr
- 2Department of Preventive Medicine, Konyang University College of Medicine, Daejeon, Korea.
- KMID: 2224974
- DOI: http://doi.org/10.4070/kcj.2012.42.11.747
Abstract
- BACKGROUND AND OBJECTIVES
The relationship between the positive remodeling (PR) of a coronary artery and plaque composition has been studied only in a relatively small number of study population or non-culprit lesion. We evaluated the association between coronary plaque composition and coronary artery remodeling in a relatively large number of culprit lesions.
SUBJECTS AND METHODS
The study population consisted of 325 consecutive patients with coronary artery disease that underwent intravascular ultrasound-virtual histology examination in a culprit lesion. The remodeling index (RI) was calculated as the lesion external elastic membrane (EEM) area divided by the average reference EEM area.
RESULTS
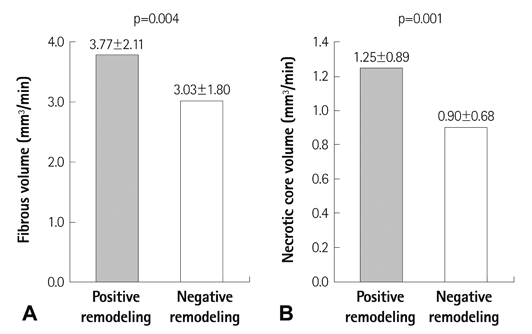
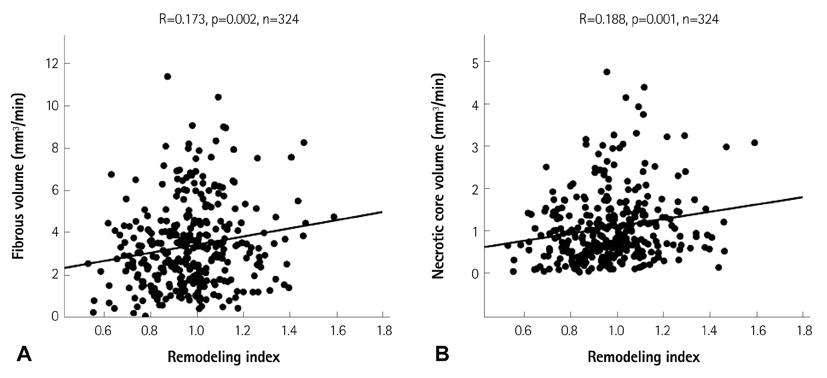
The lesions with PR (RI>1.05, n=97, mean RI=1.19+/-0.12) had a higher fibrous volume/lesion length (3.85+/-2.12 mm3/mm vs. 3.04+/-1.79 mm3/mm, p=0.003) and necrotic core volume/lesion length (1.26+/-0.89 mm3/mm vs. 0.90+/-0.66 mm3/mm, p=0.001) than those with negative remodeling (NR) (RI<0.95, n=132, mean RI=0.82+/-0.09). At the minimal luminal area site, the lesions with PR had a higher fibrous area (5.81+/-3.17 mm2 vs. 3.61+/-2.30 mm2, p<0.001), dense calcified area (0.73+/-0.69 mm2 vs. 0.46+/-0.43 mm2, p=0.001), and necrotic core area (1.93+/-1.33 mm2 vs. 1.06+/-0.91 mm2, p<0.001) than those with NR. RI showed significant positive correlation with fibrous volume/lesion length (r=0.173, p=0.002), necrotic core volume/lesion length (r=0.188, p=0.001), fibrous area (r=0.347, p<0.001), fibrofatty area (r=0.111, p=0.036), dense calcified area (r=0.239, p<0.001), and necrotic core area (r=0.334, p<0.001). Multivariate analysis showed that the independent factor for PR was the necrotic core volume/lesion length (beta=0.130, 95% confidence interval; 0.002-0.056, p=0.037) over the entire lesion.
CONCLUSION
This study suggests that PR in a culprit lesion is associated with the necrotic core volume in the entire lesion, which is a characteristic of vulnerable plaque.
MeSH Terms
Figure
Reference
-
1. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987. 316:1371–1375.2. Mintz GS, Kent KM, Pichard AD, Satler LF, Popma JJ, Leon MB. Contribution of inadequate arterial remodeling to the development of focal coronary artery stenoses. An intravascular ultrasound study. Circulation. 1997. 95:1791–1798.3. Hirose M, Kobayashi Y, Mintz GS, et al. Correlation of coronary arterial remodeling determined by intravascular ultrasound with angiographic diameter reduction of 20% to 60%. Am J Cardiol. 2003. 92:141–145.4. Nakamura M, Nishikawa H, Mukai S, et al. Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol. 2001. 37:63–69.5. Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation. 2000. 101:598–603.6. Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002. 105:939–943.7. Hiro T, Leung CY, De Guzman S, et al. Are soft echoes really soft? Intravascular ultrasound assessment of mechanical properties in human atherosclerotic tissue. Am Heart J. 1997. 133:1–7.8. Jeremias A, Kolz ML, Ikonen TS, et al. Feasibility of in vivo intravascular ultrasound tissue characterization in the detection of early vascular transplant rejection. Circulation. 1999. 100:2127–2130.9. Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006. 47:2405–2412.10. Nair A, Margolis MP, Kuban BD, Vince DG. Automated coronary plaque characterisation with intravascular ultrasound backscatter: ex vivo validation. EuroIntervention. 2007. 3:113–120.11. Fujii K, Carlier SG, Mintz GS, et al. Association of plaque characterization by intravascular ultrasound virtual histology and arterial remodeling. Am J Cardiol. 2005. 96:1476–1483.12. Rodriguez-Granillo GA, Serruys PW, Garcia-Garcia HM, et al. Coronary artery remodelling is related to plaque composition. Heart. 2006. 92:388–391.13. Surmely JF, Nasu K, Fujita H, et al. Association of coronary plaque composition and arterial remodelling: a virtual histology analysis by intravascular ultrasound. Heart. 2007. 93:928–932.14. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001. 37:1478–1492.15. Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002. 105:297–303.16. Bae JH, Kwon TG, Kim KH, Hyun DW, Kim KY, Kim DS. In-vivo coronary plaque composition in patients with acute coronary syndrome: a virtual histology intravascular ultrasound study. Korean Circ J. 2007. 37:437–442.17. Carlier SG, Mintz GS, Stone GW. Imaging of atherosclerotic plaque using radiofrequency ultrasound signal processing. J Nucl Cardiol. 2006. 13:831–840.18. Frutkin AD, Mehta SK, McCrary JR, Marso SP. Limitations to the use of virtual histology-intravascular ultrasound to detect vulnerable plaque. Eur Heart J. 2007. 28:1783–1784.19. Granada JF, Wallace-Bradley D, Win HK, et al. In vivo plaque characterization using intravascular ultrasound-virtual histology in a porcine model of complex coronary lesions. Arterioscler Thromb Vasc Biol. 2007. 27:387–393.20. Alfonso F, Hernando L. Intravascular ultrasound tissue characterization. I like the rainbow but...what's behind the colours? Eur Heart J. 2008. 29:1701–1703.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lesion Characteristics in Patients with Acute Coronary Syndrome: A Comparison with Lesion in Patients with Stable Angina by Intravascular Ultrasound
- Plaque Characteristics and Clinical Presentation Associated with Coronary Artery Remodeling: An Intravascular Ultrasound Study
- Roles of Intravascular Ultrasound in Patients with Acute Myocardial Infarction
- Effect of Adiponectin and Resistin on Coronary Plaque Composition and Coronary Artery Remodeling of Target Lesion in Patients with Stable Angina
- Multimodality Intravascular Imaging Assessment of Plaque Erosion versus Plaque Rupture in Patients with Acute Coronary Syndrome