Korean Circ J.
2013 Apr;43(4):223-230. 10.4070/kcj.2013.43.4.223.
Spatial and Temporal Expression, and Statin Responsiveness of Galectin-1 and Galectin-3 in Murine Atherosclerosis
- Affiliations
-
- 1Cardiovascular Research Center, Seoul St. Mary's Hospital, Seoul, Korea. kiyuk@catholic.ac.kr
- 2Department of Cardiovascular Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2224944
- DOI: http://doi.org/10.4070/kcj.2013.43.4.223
Abstract
- BACKGROUND AND OBJECTIVES
Existing data on the spatiotemporal expression patterns of a variety of galectins in murine atherosclerosis are limited. We investigated the expression levels of galectins, and their in vivo spatiotemporal expression patterns and statin responsiveness in the inflamed atherosclerotic plaques of apolipoprotein E (apoE)-/- mice.
MATERIALS AND METHODS
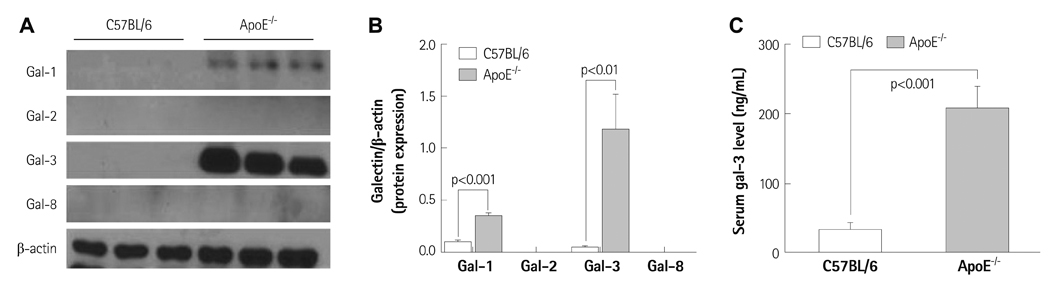
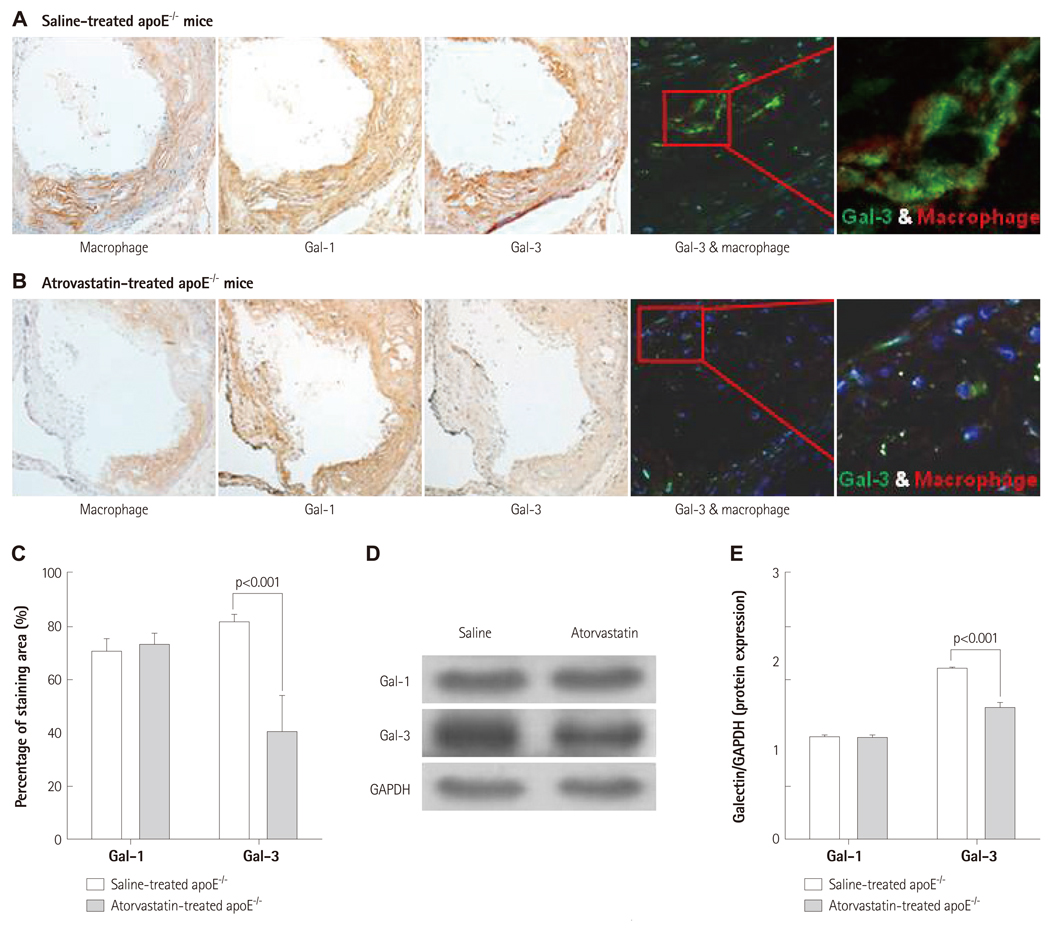
Galectins expression patterns in aortic atherosclerotic plaques and serum galectin-3 levels were investigated in 26-week-old apoE-/- (n=6) and C57BL/6 mice (n=9). To investigate the spatial and temporal patterns of galectin-1 and galectin-3 in plaques, high-cholesterol diet-fed 26-week-old (n=12) and 36-week-old apoE-/- mice (n=6) were sacrificed and their aortas were examined for galectins' expression using immunoblot analysis and immunohistochemical stain. 36-week-old apoE-/- mice were treated with atorvastatin (n=3, 0.57 mg/kg/day) for the evaluation of its effect on aortic galectins' expression.
RESULTS
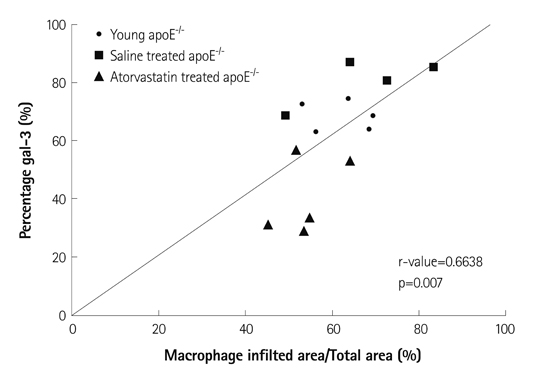
Immunoblot analyses showed that galectin-1 and galectin-3 were the predominant galectins expressed in murine atherosclerosis. The serum galectin-3 level was significantly higher in apoE-/- mice (p<0.001). While galectin-1 was weakly expressed in both intimal plaques and the media of atherosclerotic aortas, galectin-3 was heavily and exclusively accumulated in intimal plaques. Galectin-3 distribution was colocalized with plaque macrophages' distribution (r=0.66). As the degree of plaque extent and inflammation increased, the intraplaque galectin-3 expression levels proportionally elevated (p<0.01 vs. baseline), whereas galectin-1 expression had not elevated (p=0.14 vs. baseline). Atorvastatin treatment markedly reduced intraplaque galectin-3 and macrophage signals (p<0.001 vs. baseline), whereas it failed to reduce galectin-1 expression in the aortas.
CONCLUSION
Galectin-3 is the predominant gal and is colocalized with macrophages within atherosclerotic plaques. Intraplaque galectin-3 expression reflects the degree of plaque inflammation.
Keyword
MeSH Terms
Figure
Reference
-
1. Libby P, Ridker PM, Hansson GK;. Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009. 54:2129–2138.2. Almkvist J, Karlsson A. Galectins as inflammatory mediators. Glycoconj J. 2004. 19:575–581.3. Rubinstein N, Ilarregui JM, Toscano MA, Rabinovich GA. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens. 2004. 64:1–12.4. Zanetta JP, Badache A, Maschke S, Marschal P, Kuchler S. Carbohydrates and soluble lectins in the regulation of cell adhesion and proliferation. Histol Histopathol. 1994. 9:385–412.5. Rabinovich GA, Toscano MA, Ilarregui JM, Rubinstein N. Shedding light on the immunomodulatory properties of galectins: novel regulators of innate and adaptive immune responses. Glycoconj J. 2004. 19:565–573.6. Moiseeva EP, Javed Q, Spring EL, de Bono DP. Galectin 1 is involved in vascular smooth muscle cell proliferation. Cardiovasc Res. 2000. 45:493–502.7. Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999. 1473:172–185.8. Sano H, Hsu DK, Yu L, et al. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000. 165:2156–2164.9. Iacobini C, Menini S, Ricci C, et al. Accelerated lipid-induced atherogenesis in galectin-3-deficient mice: role of lipoxidation via receptormediated mechanisms. Arterioscler Thromb Vasc Biol. 2009. 29:831–836.10. Falcone C, Lucibello S, Mazzucchelli I, et al. Galectin-3 plasma levels and coronary artery disease: a new possible biomarker of acute coronary syndrome. Int J Immunopathol Pharmacol. 2011. 24:905–913.11. Kircher MF, Grimm J, Swirski FK, et al. Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation. 2008. 117:388–395.12. Rabinovich GA, Baum LG, Tinari N, et al. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002. 23:313–320.13. Chen HY, Fermin A, Vardhana S, et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci U S A. 2009. 106:14496–14501.14. MacKinnon AC, Farnworth SL, Hodkinson PS, et al. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008. 180:2650–2658.15. Jeon SB, Yoon HJ, Chang CY, Koh HS, Jeon SH, Park EJ. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol. 2010. 185:7037–7046.16. Forsman H, Islander U, Andréasson E, et al. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 2011. 63:445–454.17. Nachtigal M, Al-Assaad Z, Mayer EP, Kim K, Monsigny M. Galectin-3 expression in human atherosclerotic lesions. Am J Pathol. 1998. 152:1199–1208.18. Ozaki K, Inoue K, Sato H, et al. Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-alpha secretion in vitro. Nature. 2004. 429:72–75.19. Chellan B, Narayani J, Appukuttan PS. Galectin-1, an endogenous lectin produced by arterial cells, binds lipoprotein(a) [Lp(a)] in situ: relevance to atherogenesis. Exp Mol Pathol. 2007. 83:399–404.20. Nachtigal M, Ghaffar A, Mayer EP. Galectin-3 gene inactivation reduces atherosclerotic lesions and adventitial inflammation in ApoE-deficient mice. Am J Pathol. 2008. 172:247–255.21. Khallou-Laschet J, Varthaman A, Fornasa G, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010. 5:e8852.22. Liu FT, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010. 1183:158–182.23. Romaniuk MA, Tribulatti MV, Cattaneo V, et al. Human platelets express and are activated by galectin-8. Biochem J. 2010. 432:535–547.24. Liu FT, Yang RY, Hsu DK. Galectins in acute and chronic inflammation. Ann N Y Acad Sci. 2012. 1253:80–91.25. Arar C, Gaudin JC, Capron L, Legrand A. Galectin-3 gene (LGALS3) expression in experimental atherosclerosis and cultured smooth muscle cells. FEBS Lett. 1998. 430:307–311.26. Moiseeva EP, Williams B, Samani NJ. Galectin 1 inhibits incorporation of vitronectin and chondroitin sulfate B into the extracellular matrix of human vascular smooth muscle cells. Biochim Biophys Acta. 2003. 1619:125–132.27. Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR Jr. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995. 147:1016–1028.28. Puato M, Faggin E, Rattazzi M, et al. Atorvastatin reduces macrophage accumulation in atherosclerotic plaques: a comparison of a nonstatin-based regimen in patients undergoing carotid endarterectomy. Stroke. 2010. 41:1163–1168.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Significance of Immunohistochemical Staining in Thyroid Nodule: TPO and Galectin-3
- The Expression of Galectin-3, a Beta-Galactoside Binding Protein, in Dendritic Cells
- Effects of Intermittent Fasting on Splenic Galectin-3 Protein Expression in High-fat Diet-fed Mice
- Is the expression of p16INK4A and galectin-3 correlated with disease progression of cervical neoplasia?
- Immunohistochemical localization of galectin-3 in the brain with Theiler's murine encephalomyelitis virus (DA strain) infection