Korean Circ J.
2013 Jun;43(6):377-383. 10.4070/kcj.2013.43.6.377.
Impact of Plaque Composition on Long-Term Clinical Outcomes in Patients with Coronary Artery Occlusive Disease
- Affiliations
-
- 1Division of Cardiology, Konyang University Hospital, Daejeon, Korea. janghobae@yahoo.co.kr
- 2Division of Cardiology, Andong Sungso Hospital, Andong, Korea.
- 3Division of Cardiology, Mayo Clinic, Rochester, MN, USA.
- 4Division of Epidemiology, Konyang University, Daejeon, Korea.
- KMID: 2224898
- DOI: http://doi.org/10.4070/kcj.2013.43.6.377
Abstract
- BACKGROUND AND OBJECTIVES
It is unclear which plaque component is related with long-term clinical outcomes in patients with coronary artery occlusive disease (CAOD). We assessed the relationship between plaque compositions and long-term clinical outcomes in those patients.
SUBJECTS AND METHODS
The study subjects consisted of 339 consecutive patients (mean 61.7+/-12.2 years old, 239 males) who underwent coronary angiogram and a virtual histology-intravascular ultrasound examination. Major adverse cardiac and cerebrovascular events (MACCE), including all-cause death, non-fatal myocardial infarction, cerebrovascular events, and target vessel revascularization were evaluated during a mean 28-month follow-up period.
RESULTS
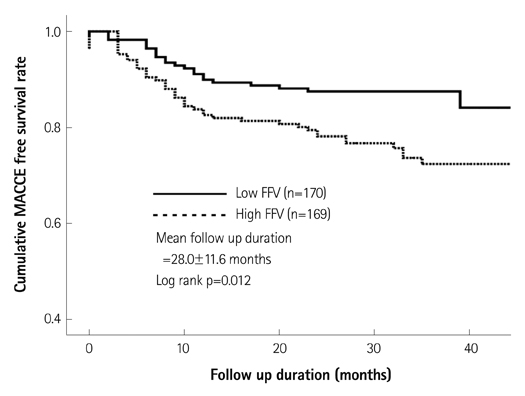
Patients with high fibrofatty volume (FFV, >8.90 mm3, n=169) had a higher incidence of MACCE (25.4% vs. 14.7%, p=0.015), male sex (75.7% vs. 65.3%, p=0.043), acute coronary syndrome (53.3% vs. 35.9%, p=0.002), multivessel disease (62.7% vs. 41.8%, p<0.001) and post-stent slow flow (10.7% vs. 2.4%, p=0.002) than those with low FFV (FFV< or =8.90 mm3, n=170). Other plaque composition factors such as fibrous area/volume, dense calcified area/volume, and necrotic core area/volume did not show any impact on MACCE. Cardiogenic shock {hazard ratio (HR)=8.44; 95% confidence interval (CI)=3.00-23.79; p<0.001} and FFV (HR=1.85; 95% CI=1.12-3.07; p=0.016) were the independent predictors of MACCE by Cox regression analysis. Thin-cap fibroatheroma, necrotic core area, and necrotic core volume were not associated with MACCE.
CONCLUSION
FFV of a culprit lesion was associated with unfavorable long-term clinical outcomes in patients with CAOD.
MeSH Terms
Figure
Reference
-
1. Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003; 41:4 Suppl S. 15S–22S.2. DeMaria AN, Narula J, Mahmud E, Tsimikas S. Imaging vulnerable plaque by ultrasound. J Am Coll Cardiol. 2006; 47:8 Suppl. C32–C39.3. Fujii K, Carlier SG, Mintz GS, et al. Association of plaque characterization by intravascular ultrasound virtual histology and arterial remodeling. Am J Cardiol. 2005; 96:1476–1483.4. Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002; 106:2200–2206.5. Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006; 47:2405–2412.6. Rodriguez-Granillo GA, McFadden EP, Valgimigli M, et al. Coronary plaque composition of nonculprit lesions, assessed by in vivo intracoronary ultrasound radio frequency data analysis, is related to clinical presentation. Am Heart J. 2006; 151:1020–1024.7. Rodriguez-Granillo GA, Serruys PW, Garcia-Garcia HM, et al. Coronary artery remodelling is related to plaque composition. Heart. 2006; 92:388–391.8. Nair A, Margolis MP, Kuban BD, Vince DG. Automated coronary plaque characterisation with intravascular ultrasound backscatter: ex vivo validation. EuroIntervention. 2007; 3:113–120.9. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011; 364:226–235.10. Marso SP, Mercado N, Maehara A, et al. Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc Imaging. 2012; 5:3 Suppl. S42–S52.11. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012; 126:2020–2035.12. Kim WH, Park HW, Kim KH, et al. Fibro-fatty component is important for the long-term clinical events in patients who have undergone primary percutaneous coronary intervention. Korean Circ J. 2012; 42:33–39.13. Bae JH, Kwon TG, Hyun DW, Rihal CS, Lerman A. Predictors of slow flow during primary percutaneous coronary intervention: an intravascular ultrasound-virtual histology study. Heart. 2008; 94:1559–1564.14. Nakamura T, Kubo N, Ako J, Momomura S. Angiographic no-reflow phenomenon and plaque characteristics by virtual histology intravascular ultrasound in patients with acute myocardial infarction. J Interv Cardiol. 2007; 20:335–339.15. Surmely JF, Nasu K, Fujita H, et al. Association of coronary plaque composition and arterial remodelling: a virtual histology analysis by intravascular ultrasound. Heart. 2007; 93:928–932.16. Missel E, Mintz GS, Carlier SG, et al. In vivo virtual histology intravascular ultrasound correlates of risk factors for sudden coronary death in men: results from the prospective, multi-centre virtual histology intravascular ultrasound registry. Eur Heart J. 2008; 29:2141–2147.17. Philipp S, Böse D, Wijns W, et al. Do systemic risk factors impact invasive findings from virtual histology? Insights from the international virtual histology registry. Eur Heart J. 2010; 31:196–202.18. Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002; 105:297–303.19. Fuessl RT, Kranenberg E, Kiausch U, Baer FM, Sechtem U, Höpp HW. Vascular remodeling in atherosclerotic coronary arteries is affected by plaque composition. Coron Artery Dis. 2001; 12:91–97.20. Sabaté M, Kay IP, de Feyter PJ, et al. Remodeling of atherosclerotic coronary arteries varies in relation to location and composition of plaque. Am J Cardiol. 1999; 84:135–140.21. Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004; 110:3424–3429.22. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000; 20:1262–1275.23. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995; 92:657–671.24. Silva JA, Escobar A, Collins TJ, Ramee SR, White CJ. Unstable angina. A comparison of angioscopic findings between diabetic and nondiabetic patients. Circulation. 1995; 92:1731–1736.25. Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995; 91:2844–2850.26. Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993; 69:377–381.27. Buja LM, Willerson JT. Role of inflammation in coronary plaque disruption. Circulation. 1994; 89:503–505.28. Carlier SG, Mintz GS, Stone GW. Imaging of atherosclerotic plaque using radiofrequency ultrasound signal processing. J Nucl Cardiol. 2006; 13:831–840.29. Frutkin AD, Mehta SK, McCrary JR, Marso SP. Limitations to the use of virtual histology-intravascular ultrasound to detect vulnerable plaque. Eur Heart J. 2007; 28:1783–1784.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Coronary Physiology-Based Approaches for Plaque Vulnerability: Implications for Risk Prediction and Treatment Strategies
- Carotid ultrasound in patients with coronary artery disease
- Statin Therapy with Coronary Plaque Imaging
- Coronary Artery Imaging in Children
- Comparison of Plaque Composition in Diabetic and Non-Diabetic Patients With Coronary Artery Disease Using Multislice CT Angiography