Korean Circ J.
2014 Mar;44(2):105-112. 10.4070/kcj.2014.44.2.105.
Nitric Oxide Synthase Inhibition Attenuates Cardiac Response to Hemodilution with Viscogenic Plasma Expander
- Affiliations
-
- 1Institute of Biomedical Engineering, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand. surapong.c@psu.ac.th
- 2Department of Bioengineering, University of California, San Diego, CA, USA.
- KMID: 2223899
- DOI: http://doi.org/10.4070/kcj.2014.44.2.105
Abstract
- BACKGROUND AND OBJECTIVES
Increased vascular wall shear stress by elevated plasma viscosity significantly enhances the endothelial nitric oxide synthase (eNOS) activity during an acute isovolemic hemodilution. Also the modulation of plasma viscosity has effects on the cardiac function that were revealed if a left ventricular (LV) pressure-volume (PV) measurement was used. The aim of this study was to assess cardiac function responses to nitric oxide synthase (NOS) inhibitors with the presence of an elevated plasma viscosity but a low hematocrit level. Furthermore, systemic parameters were monitored in a murine model.
MATERIALS AND METHODS
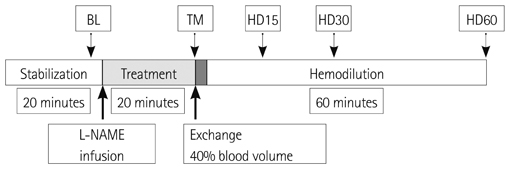
As test group five anesthetized hamsters were administered with N(G)-nitro-L-arginine methyl ester (L-NAME), NOS inhibitor, whereas five other hamsters were used as control group without L-NAME infusion. The dosage of L-NAME was 10 mg/kg. An isovolemic hemodilution was performed by 40% of estimated blood volume with 6% w/v dextran 2000 kDa, high viscosity plasma expanders (PEs) with viscosity 6.34 cP. LV function was measured and assessed using a 1.4 Fr PV conductance catheter.
RESULTS
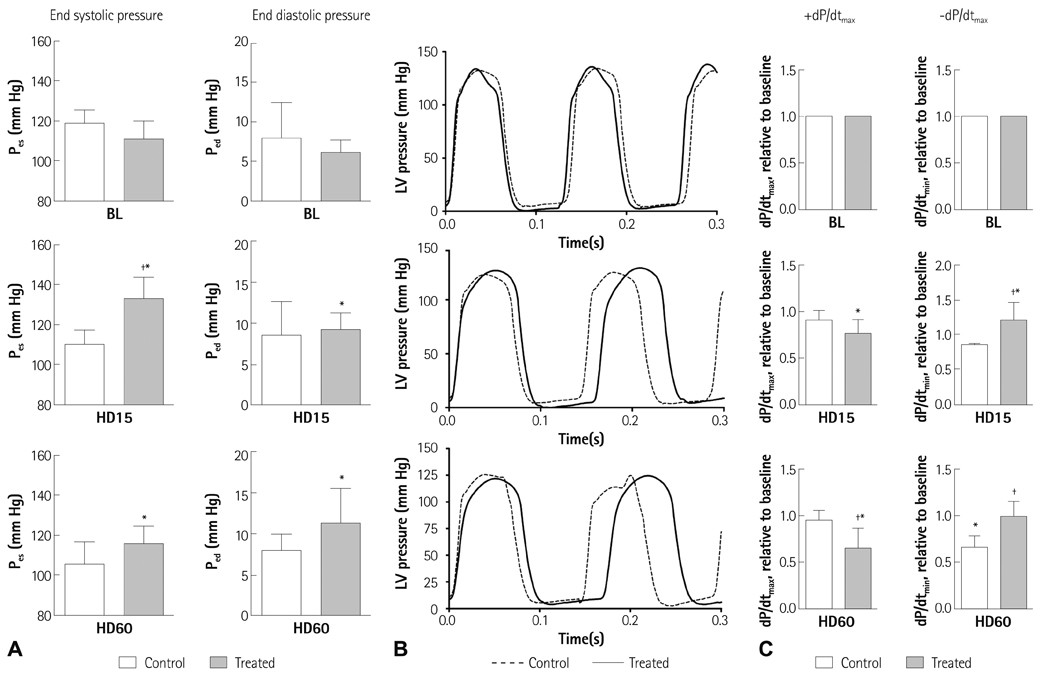
The study results demonstrated that NOS inhibition prevented the normal cardiac adaptive response after hemodilution. The endsystolic pressure increased 14% after L-NAME infusion and maintained higher than at the baseline after hemodilution, whereas it gradually decreased in the animals without L-NAME infusion. The admission of L-NAME significantly decreased the maximum rate of ventricular pressure rise (+dP/dtmax), stroke volume and cardiac output after hemodilution if compared to the control group (p<0.05).
CONCLUSION
This finding supports the presumption that nitric oxide induced by an increased plasma viscosity with the use of a high viscosity PE plays a major role in the cardiac function during an acute isovolemic hemodilution.
Keyword
MeSH Terms
-
Animals
Blood Viscosity
Blood Volume
Cardiac Output
Catheters
Cricetinae
Dextrans
Hematocrit
Hemodilution*
NG-Nitroarginine Methyl Ester
Nitric Oxide Synthase Type III
Nitric Oxide Synthase*
Nitric Oxide*
Plasma*
Stroke Volume
Ventricular Function
Ventricular Pressure
Viscosity
Dextrans
NG-Nitroarginine Methyl Ester
Nitric Oxide
Nitric Oxide Synthase
Nitric Oxide Synthase Type III
Figure
Reference
-
1. Boo YC. Shear stress stimulates phosphorylation of protein kinase A substrate proteins including endothelial nitric oxide synthase in endothelial cells. Exp Mol Med. 2006; 38:453.2. Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res. 1996; 79:984–991.3. Shesely EG, Maeda N, Kim HS, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996; 93:13176–13181.4. Rastaldo R, Pagliaro P, Cappello S, et al. Nitric oxide and cardiac function. Life Sci. 2007; 81:779–793.5. Palmer RM, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988; 153:1251–1256.6. Hsieh NK, Chang HR, Hu CT, Chen HI. Effects of nitric oxide donor and nitric oxide synthase inhibitor on the resistance, exchange and capacitance functions of the canine intestinal vasculature. Vascul Pharmacol. 2008; 48:122–128.7. Fitch RM, Vergona R, Sullivan ME, Wang YX. Nitric oxide synthase inhibition increases aortic stiffness measured by pulse wave velocity in rats. Cardiovasc Res. 2001; 51:351–358.8. Sakai H, Hara H, Tsai AG, Tsuchida E, Intaglietta M. Constriction of resistance arteries determines l-NAME-induced hypertension in a conscious hamster model. Microvasc Res. 2000; 60:21–27.9. Fozard JR, Part ML. Haemodynamic responses to NG-monomethyl-L-arginine in spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Pharmacol. 1991; 102:823–826.10. de Wit C, Schäfer C, von Bismarck P, Bolz SS, Pohl U. Elevation of plasma viscosity induces sustained NO-mediated dilation in the hamster cremaster microcirculation in vivo. Pflugers Arch. 1997; 434:354–361.11. Tsai AG, Acero C, Nance PR, et al. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol. 2005; 288:H1730–H1739.12. Cabrales P, Intaglietta M, Tsai AG. Increase plasma viscosity sustains microcirculation after resuscitation from hemorrhagic shock and continuous bleeding. Shock. 2005; 23:549–555.13. Cabrales P, Tsai AG. Plasma viscosity regulates systemic and microvascular perfusion during acute extreme anemic conditions. Am J Physiol Heart Circ Physiol. 2006; 291:H2445–H2452.14. Cabrales P, Tsai AG, Intaglietta M. Increased plasma viscosity prolongs microhemodynamic conditions during small volume resuscitation from hemorrhagic shock. Resuscitation. 2008; 77:379–386.15. Chatpun S, Cabrales P. Cardiac mechanoenergetic cost of elevated plasma viscosity after moderate hemodilution. Biorheology. 2010; 47:225–237.16. Chatpun S, Cabrales P. Exogenous intravascular nitric oxide enhances ventricular function after hemodilution with plasma expander. Life Sci. 2012; 90:39–46.17. Chatpun S, Cabrales P. Effects of plasma viscosity modulation on cardiac function during moderate hemodilution. Asian J Transfus Sci. 2010; 4:102–108.18. Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008; 3:1422–1434.19. Amezcua JL, Palmer RM, de Souza BM, Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol. 1989; 97:1119–1124.20. Pabla R, Curtis MJ. Effects of NO modulation on cardiac arrhythmias in the rat isolated heart. Circ Res. 1995; 77:984–992.21. Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990; 101:746–752.22. Pfeiffer S, Leopold E, Schmidt K, Brunner F, Mayer B. Inhibition of nitric oxide synthesis by NG-nitro-L-arginine methyl ester (L-NAME): requirement for bioactivation to the free acid, NG-nitro-L-arginine. Br J Pharmacol. 1996; 118:1433–1440.23. Ward JE, Angus JA. Acute and chronic inhibition of nitric oxide synthase in conscious rabbits: role of nitric oxide in the control of vascular tone. J Cardiovasc Pharmacol. 1993; 21:804–814.24. Bryan S, Alexander-Lindo R, McGrowder D. The effect of nitric oxide inhibitors and s-nitrosothiols on hemodynamic parameters in an animal model. Open Access Animal Physiology. 2011; 3:1–8.25. Crystal GJ, Zhou X, Halim AA, Alam S, El-Orbany M, Salem MR. Nitric oxide does not modulate whole body oxygen consumption in anesthetized dogs. J Appl Physiol (1985). 1999; 86:1944–1949.26. Herring N, Paterson DJ. Endothelial nitric oxide synthase and heart rate. Circulation. 2002; 106:e5. author reply e5.27. Widdop RE, Gardiner SM, Kemp PA, Bennett T. The influence of atropine and atenolol on the cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br J Pharmacol. 1992; 105:653–656.28. Biwer LA, Broderick TL, Xu H, Carroll C, Hale TM. Protection against L-NAME-induced reduction in cardiac output persists even after cessation of angiotensin-converting enzyme inhibitor treatment. Acta Physiol (Oxf). 2013; 207:156–165.29. Chen Y, Traverse JH, Du R, Hou M, Bache RJ. Nitric oxide modulates myocardial oxygen consumption in the failing heart. Circulation. 2002; 106:273–279.30. Crystal GJ, Zhou X. Nitric oxide does not modulate the increases in blood flow, O2 consumption, or contractility during CaCl2 administration in canine hearts. Cardiovasc Res. 1999; 42:232–239.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nitric Oxide Synthase Inhibition Alters Extracellular Glutamate Concentration after Global Cerebral Ischemia
- Alteration of Nitric Oxide Synthase Subtype Expression in Contralateral Testis of Rat in Response to Unilateral Testicular Torsion Followed by Detorsion
- Role of nitric oxide and distribution of nitric oxide synthase in the gustatory system
- Nitric Oxide in the Kidney: Its Physiological Role and Pathophysiological Implications
- Distribution of Nitric Oxide Synthase Isoforms in Perioral Exocrine Glands in Rats