Korean Circ J.
2014 Jul;44(4):243-249. 10.4070/kcj.2014.44.4.243.
Effects of Low-Intensity Autonomic Nerve Stimulation on Atrial Electrophysiology
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. seil@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2223885
- DOI: http://doi.org/10.4070/kcj.2014.44.4.243
Abstract
- BACKGROUND AND OBJECTIVES
The cardiac autonomic nervous system is an emerging target for therapeutic control of atrial fibrillation (AF). We evaluated the effects of low-intensity autonomic nerve stimulation (LI-ANS) on atrial electrophysiology, AF vulnerability, and neural remodeling.
SUBJECTS AND METHODS
Fourteen dogs were subjected to 3 hours rapid atrial pacing (RAP, 5 Hz) and concomitant high frequency LI-ANS (20 Hz, at voltages 40% below the threshold) as follows: no autonomic stimulation (control, n=3); or right cervical vagus nerve (RVN, n=6), anterior right ganglionated plexi (ARGP, n=3), and superior left ganglionated plexi (SLGP, n=2) stimulation. Programmed and burst atrial pacing were performed at baseline and at the end of each hour to determine atrial effective refractory period (ERP), window of vulnerability (WOV), and inducibility of sustained AF.
RESULTS
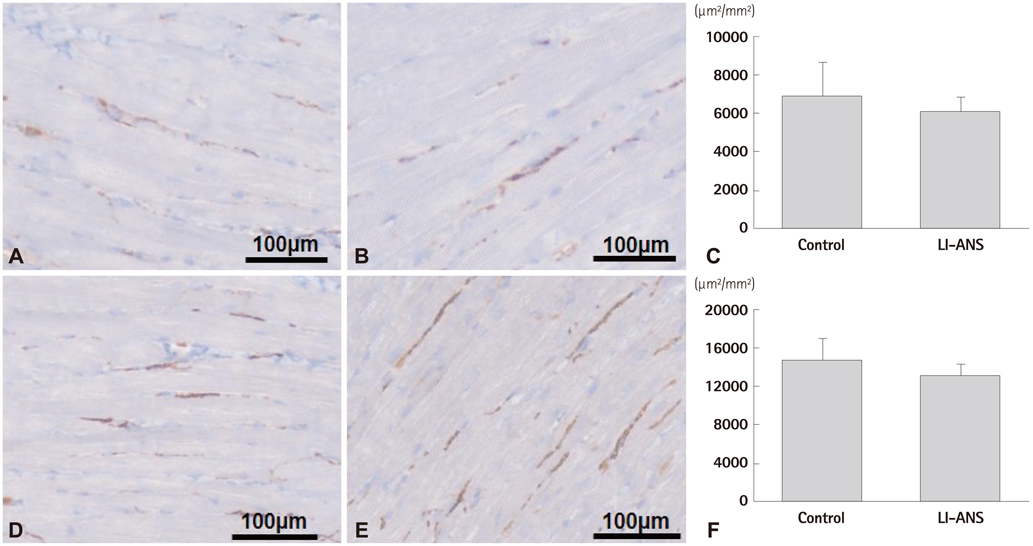
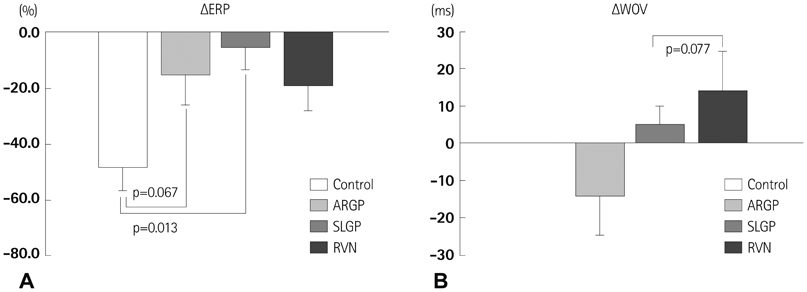
Atrial ERP was significantly shortened by 3 hours RAP (in control group, DeltaERP=-47.9+/-8.9%, p=0.032), and RAP-induced ERP shortening was attenuated by LI-ANS (in LI-ANS group, DeltaERP=-15.4+/-5.9%, p=0.019; vs. control, p=0.035). Neither WOV for AF nor AF inducibility changed significantly during 3 hours RAP with simultaneous LI-ANS. There was no significant difference between the control and LI-ANS group in nerve density and sprouting evaluated by anti-tyrosine hydroxylase and anti-growth associated protein-43 staining. Among the various sites for LI-ANS, the ARGP-stimulation group showed marginally lower DeltaWOV (p=0.077) and lower nerve sprouting (p=0.065) compared to the RVN-stimulation group.
CONCLUSION
Low-intensity autonomic nerve stimulation significantly attenuated the shortening of atrial ERP caused by RAP. ARGP may be a better target for LI-ANS than RVN for the purpose of suppressing atrial remodeling in AF.
MeSH Terms
Figure
Reference
-
1. Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011; 57:e101–e198.2. Roy D, Talajic M, Dorian P, et al. Canadian Trial of Atrial Fibrillation Investigators. Amiodarone to prevent recurrence of atrial fibrillation. N Engl J Med. 2000; 342:913–920.3. Zhang Y, Ilsar I, Sabbah HN, Ben David T, Mazgalev TN. Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm. 2009; 6:244–250.4. Tai CT, Chiou CW, Chen SA. Interaction between the autonomic nervous system and atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 2002; 13:83–87.5. Coumel P. Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 1996; 7:999–1007.6. Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002; 105:2753–2759.7. Oh S, Zhang Y, Bibevski S, Marrouche NF, Natale A, Mazgalev TN. Vagal denervation and atrial fibrillation inducibility: epicardial fat pad ablation does not have long-term effects. Heart Rhythm. 2006; 3:701–708.8. Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011; 123:2204–2212.9. Cao JM, Fishbein MC, Han JB, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000; 101:1960–1969.10. Wijffels MC, Kirchhof CJ, Dorland R, Power J, Allessie MA. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation. 1997; 96:3710–3720.11. Chang CM, Wu TJ, Zhou S, et al. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001; 103:22–25.12. Ogawa M, Tan AY, Song J, et al. Cryoablation of stellate ganglia and atrial arrhythmia in ambulatory dogs with pacing-induced heart failure. Heart Rhythm. 2009; 6:1772–1779.13. Ghias M, Scherlag BJ, Lu Z, et al. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol. 2009; 54:2075–2083.14. Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011; 8:672–678.15. Pokushalov E, Romanov A, Shugayev P, et al. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009; 6:1257–1264.16. Schauerte P, Scherlag BJ, Pitha J, et al. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000; 102:2774–2780.17. Wakili R, Voigt N, Kääb S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011; 121:2955–2968.18. Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001; 103:769–777.19. Oh S. Disease modification by autonomic nerve stimulation. Heart Rhythm. 2012; 9:810–811.20. Sheng X, Scherlag BJ, Yu L, et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol. 2011; 57:563–571.21. Prechtl JC, Powley TL. The fiber composition of the abdominal vagus of the rat. Anat Embryol (Berl). 1990; 181:101–115.22. Hoover DB, Isaacs ER, Jacques F, Hoard JL, Pagé P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience. 2009; 164:1170–1179.23. Choi EK, Shen MJ, Han S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010; 121:2615–2623.24. Zhang Y, Yamada H, Bibevski S, et al. Chronic atrioventricular nodal vagal stimulation: first evidence for long-term ventricular rate control in canine atrial fibrillation model. Circulation. 2005; 112:2904–2911.25. Sakamoto S, Schuessler RB, Lee AM, Aziz A, Lall SC, Damiano RJ Jr. Vagal denervation and reinnervation after ablation of ganglionated plexi. J Thorac Cardiovasc Surg. 2010; 139:444–452.26. Jacobson RD, Virág I, Skene JH. A protein associated with axon growth, GAP-43, is widely distributed and developmentally regulated in rat CNS. J Neurosci. 1986; 6:1843–1855.27. Goslin K, Banker G. Rapid changes in the distribution of GAP-43 correlate with the expression of neuronal polarity during normal development and under experimental conditions. J Cell Biol. 1990; 110:1319–1331.28. Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec. 1994; 239:75–87.29. Hou Y, Scherlag BJ, Lin J, et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007; 50:61–68.30. Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008; 118:916–925.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Penile erection evoked by autonomic nerve stimulation in rats
- Effects of Several Autonomic Drugs on the Responses to Electrical Stimulation of the Detrusor Muscle Strips in Amyda Japonica
- Atrial fibrillation and chronic kidney disease: A bad combination
- Alterations of Heart Rate Variability by Vestibular Stimulation in Rabbits
- Electrophysiological Basis of Fecal Incontinence and Its Implications for Treatment