Changes in Caspase-3, B Cell Leukemia/Lymphoma-2, Interleukin-6, Tumor Necrosis Factor-α and Vascular Endothelial Growth Factor Gene Expression after Human Umbilical Cord Blood Derived Mesenchymal Stem Cells Transfusion in Pulmonary Hypertension Rat Models
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Ewha Womans University School of Medicine, Seoul, Korea.
- 2Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea. ymhong@ewha.ac.kr
- 3Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea.
- 4Biomedical Research Institute, MEDIPOST, Co., Seoul, Korea.
- KMID: 2223773
- DOI: http://doi.org/10.4070/kcj.2016.46.1.79
Abstract
- BACKGROUND AND OBJECTIVES
Failure of vascular smooth muscle apoptosis and inflammatory response in pulmonary arterial hypertension (PAH) is a current research focus. The goals of this study were to determine changes in select gene expressions in monocrotaline (MCT)-induced PAH rat models after human umbilical cord blood derived mesenchymal stem cells (hUCB-MSCs) transfusion.
MATERIALS AND METHODS
The rats were separated into 3 groups i.e., control group (C group), M group (MCT 60 mg/kg), and U group (hUCB-MSCs transfusion) a week after MCT injection.
RESULTS
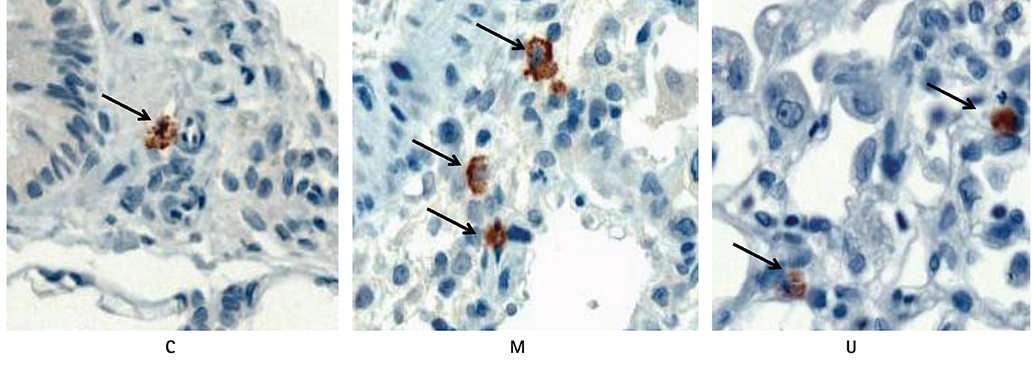
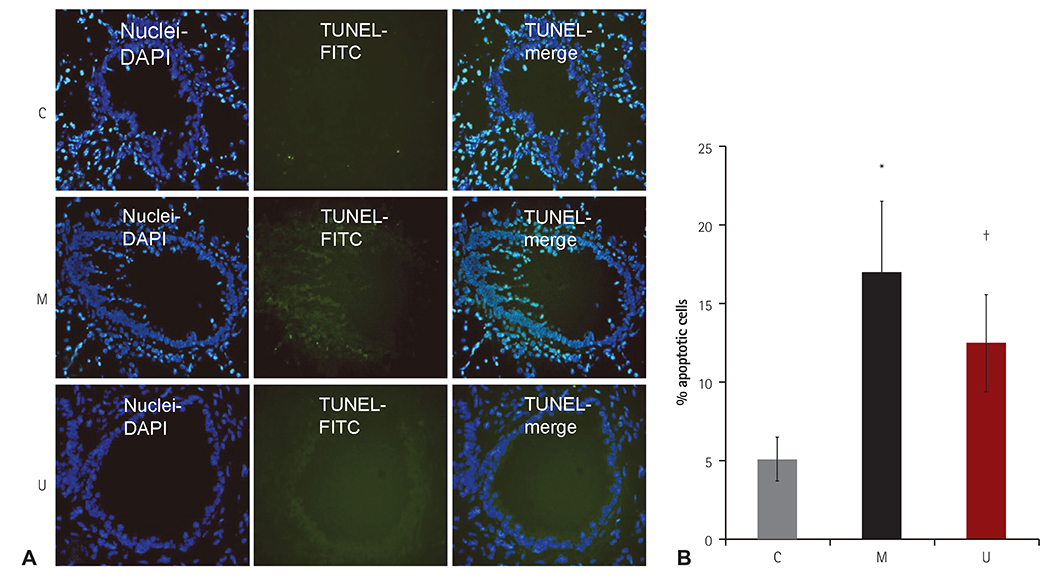
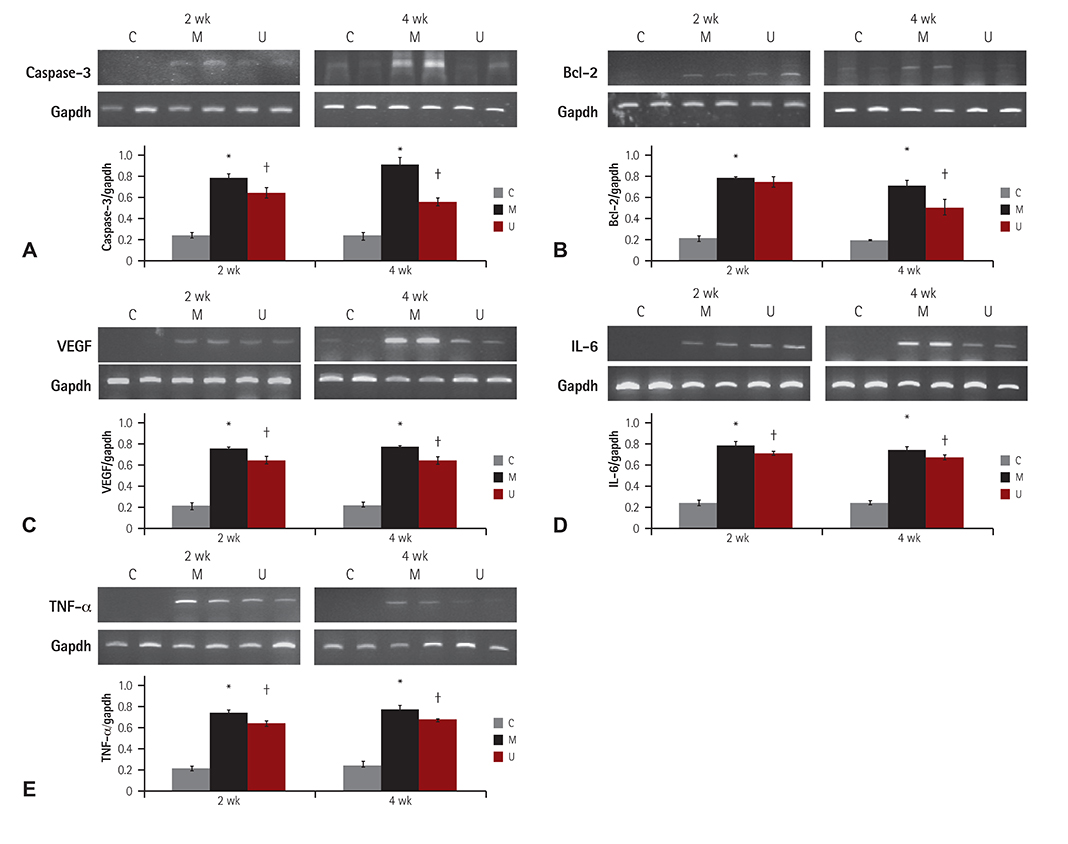
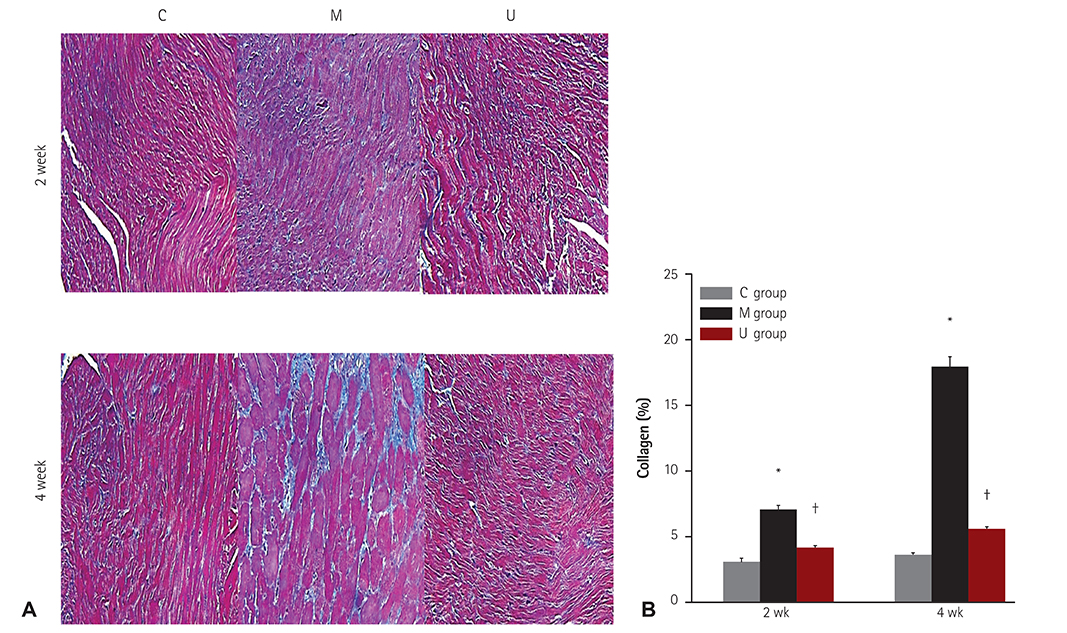
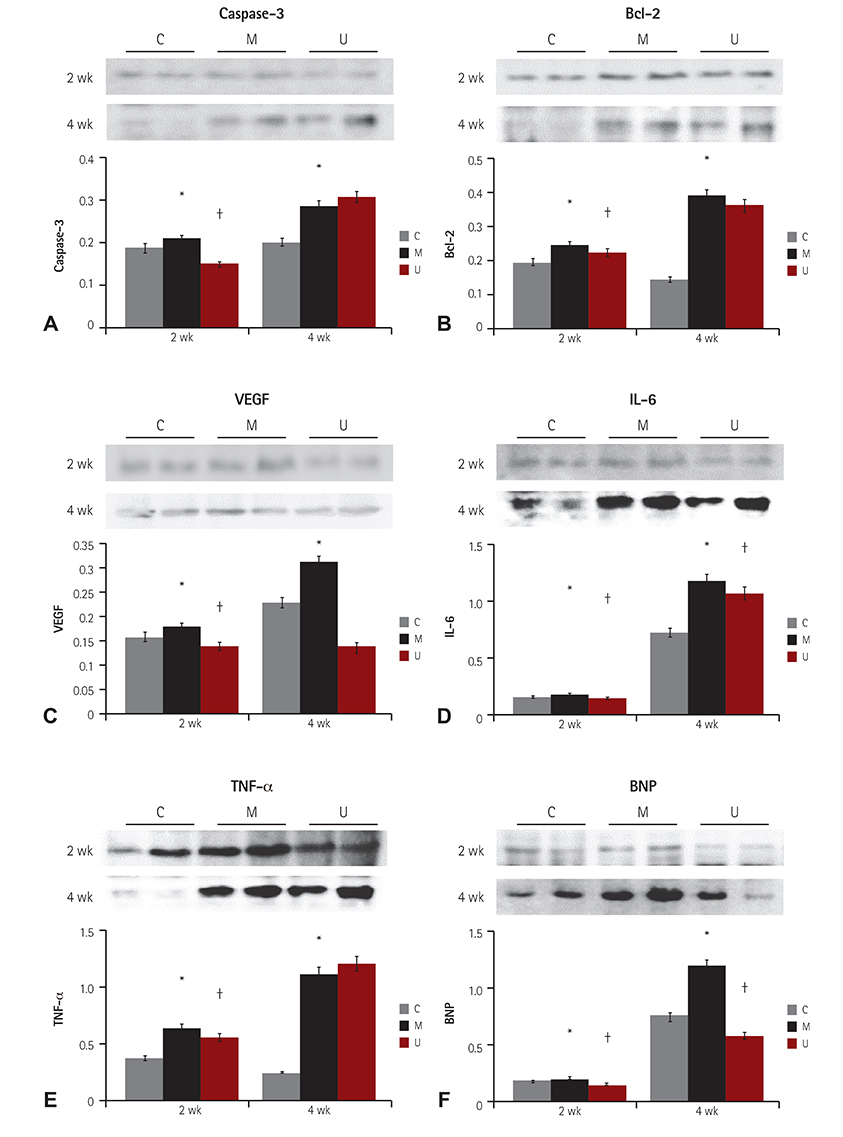
TUNEL assay showed that the U group had significantly lowered positive apoptotic cells in the lung tissues, as compared with the M group. mRNA of caspase-3, B cell leukemia/lymphoma (Bcl)-2, interleukin (IL)-6, tumor necrosis factor (TNF)-alpha and vascular endothelial growth factor (VEGF) in the lung tissues were greatly reduced at week 4 in the U group. Immunohistochemical staining of the lung tissues also demonstrated a similar pattern, with the exception of IL-6. The protein expression of caspase-3, Bcl-2 VEGF, IL-6, TNF-alpha and brain natriuretic peptide in the heart tissues were significantly lower in the U group, as compared with the M group at week 2. Furthermore, the protein expression of VEGF, IL-6 and BNP in the heart tissues were significantly lower in the U group at week 4. Collagen content in the heart tissues was significantly lower in the U group, as compared with M group at weeks 2 and 4, respectively.
CONCLUSION
hUCB-MSCs could prevent inflammation, apoptosis and remodeling in MCT-induced PAH rat models.
MeSH Terms
-
Animals
Apoptosis
Caspase 3*
Collagen
Fetal Blood*
Gene Expression*
Heart
Humans*
Hypertension
Hypertension, Pulmonary*
In Situ Nick-End Labeling
Inflammation
Interleukin-6*
Interleukins
Lung
Mesenchymal Stromal Cells*
Models, Animal*
Monocrotaline
Muscle, Smooth, Vascular
Natriuretic Peptide, Brain
Rats*
RNA, Messenger
Stem Cells
Tumor Necrosis Factor-alpha*
Umbilical Cord*
Vascular Endothelial Growth Factor A*
Caspase 3
Collagen
Interleukin-6
Interleukins
Monocrotaline
Natriuretic Peptide, Brain
RNA, Messenger
Tumor Necrosis Factor-alpha
Vascular Endothelial Growth Factor A
Figure
Cited by 4 articles
-
New Therapeutic Target for Pulmonary Arterial Hypertension
Kyung Lim Yoon
Korean Circ J. 2018;48(12):1145-1147. doi: 10.4070/kcj.2018.0250.Benefits from the Early Initiation of Macitentan for Pulmonary Arterial Hypertension
Hae Ok Jung
Korean Circ J. 2018;48(9):854-856. doi: 10.4070/kcj.2018.0156.Effect of Ambrisentan Therapy on the Expression of Endothelin Receptor, Endothelial Nitric Oxide Synthase and NADPH Oxidase 4 in Monocrotaline-induced Pulmonary Arterial Hypertension Rat Model
Hyeryon Lee, Arim Yeom, Kwan Chang Kim, Young Mi Hong
Korean Circ J. 2019;49(9):866-876. doi: 10.4070/kcj.2019.0006.Optimal Dose and Timing of Umbilical Stem Cells Treatment in Pulmonary Arterial Hypertensive Rats
Hyeryon Lee, Kwan Chang Kim, Soo Jin Choi, Young Mi Hong
Yonsei Med J. 2017;58(3):570-580. doi: 10.3349/ymj.2017.58.3.570.
Reference
-
1. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004; 109:159–165.2. Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther. 2010; 126:1–8.3. Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis. 2002; 45:173–202.4. Sage E, Mercier O, Van den Eyden F, et al. Endothelial cell apoptosis in chronically obstructed and reperfused pulmonary artery. Respir Res. 2008; 9:19.5. Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005; 19:1178–1180.6. Thomas HC, Lamé MW, Dunston SK, Segall HJ, Wilson DW. Monocrotaline pyrrole induces apoptosis in pulmonary artery endothelial cells. Toxicol Appl Pharmacol. 1998; 151:236–244.7. Price LC, Wort SJ, Perros F, et al. Inflammation in pulmonary arterial hypertension. Chest. 2012; 141:210–221.8. Perros F, Dorfmüller P, Souza R, et al. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J. 2007; 29:462–468.9. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009; 297:L1013–L1032.10. Lévy M, Maurey C, Celermajer DS, et al. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol. 2007; 49:803–810.11. Han I, Yun M, Kim EO, Kim B, Jung MH, Kim SH. Umbilical cord tissue-derived mesenchymal stem cells induce apoptosis in PC-3 prostate cancer cells through activation of JNK and downregulation of PI3K/AKT signaling. Stem Cell Res Ther. 2014; 5:54.12. Ohnishi S, Yanagawa B, Tanaka K, et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007; 42:88–97.13. Semedo P, Wang PM, Andreucci TH, et al. Mesenchymal stem cells ameliorate tissue damages triggered by renal ischemia and reperfusion injury. Transplant Proc. 2007; 39:421–423.14. Lee H, Lee JC, Kwon JH, et al. The effect of umbilical cord blood derived mesenchymal stem cells in monocrotaline-induced pulmonary artery hypertension rats. J Korean Med Sci. 2015; 30:576–585.15. Puri MC, Nagy A. Concise review: embryonic stem cells versus induced pluripotent stem cells: the game is on. Stem Cells. 2012; 30:10–14.16. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006; 24:1294–1301.17. Hong YM, Kwon JH, Choi S, Kim KC. Apoptosis and inflammation associated gene expressions in monocrotaline-induced pulmonary hypertensive rats after bosentan treatment. Korean Circ J. 2014; 44:97–104.18. Kim KC, Lee HR, Kim SJ, Cho MS, Hong YM. Changes of gene expression after bone marrow cell transfusion in rats with monocrotaline-induced pulmonary hypertension. J Korean Med Sci. 2012; 27:605–613.19. Chu PH, Jung SM, Yeh CH, et al. Expression of caspase-3-dependent apoptosis in mural thrombi leukocytes. APMIS. 2008; 116:995–999.20. Cai J, Jiang WG, Ahmed A, Boulton M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc Res. 2006; 71:20–31.21. Grosjean J, Kiriakidis S, Reilly K, Feldmann M, Paleolog E. Vascular endothelial growth factor signalling in endothelial cell survival: a role for NFkappaB. Biochem Biophys Res Commun. 2006; 340:984–994.22. Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009; 135:794–804.23. Krown KA, Page MT, Nguyen C, et al. Tumor necrosis factor-induced apoptosis in cardiac myocytes: involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996; 98:2854–2865.24. Kret M, Arora R. Pathophysiological basis of right ventricular remodeling. J Cardiovasc Pharmacol Ther. 2007; 12:5–14.25. Küçüker SA, Stetson SJ, Becker KA, et al. Evidence of improved right ventricular structure after LVAD support in patients with end-stage cardiomyopathy. J Heart Lung Transplant. 2004; 23:28–35.26. Ryan JJ, Huston J, Kutty S, et al. Right ventricular adaptation and failure in pulmonary arterial hypertension. Can J Cardiol. 2015; 31:391–406.27. Lee JC, Kim KC, Yang YS, et al. Microarray analysis after umbilical cord blood derived mesenchymal stem cells injection in monocrotaline-induced pulmonary artery hypertension rats. Anat Cell Biol. 2014; 47:217–226.28. Chang YS, Oh W, Choi SJ. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009; 18:869–886.29. Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Stem Cell Mol Biol. 2005; 33:145–152.30. Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003; 100:8407–8411.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- Apoptosis and Inflammation Associated Gene Expressions in Monocrotaline-Induced Pulmonary Hypertensive Rats after Bosentan Treatment
- Difference in Cell Characteristics among the Monoclonal Cell Populations Obtained from the Human Umbilical Cord Blood Derived Mesenchymal Stem Cell Population
- Effects of magnesium sulfate on VEGF and Caspase-3 of HUVECs (Human umbilical vein endothelial cells)
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood