J Rheum Dis.
2013 Apr;20(2):108-112. 10.4078/jrd.2013.20.2.108.
Rituximab for Rheumatoid Arthritis Following TNF-alpha Inhibitor Associated Splenic Tuberculosis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Chung-Ang University School of Medicine, Seoul, Korea. beconst@cau.ac.kr
- 2Department of Internal Medicine, Pohang St. Mary Hospital, Pohang, Korea.
- KMID: 2223035
- DOI: http://doi.org/10.4078/jrd.2013.20.2.108
Abstract
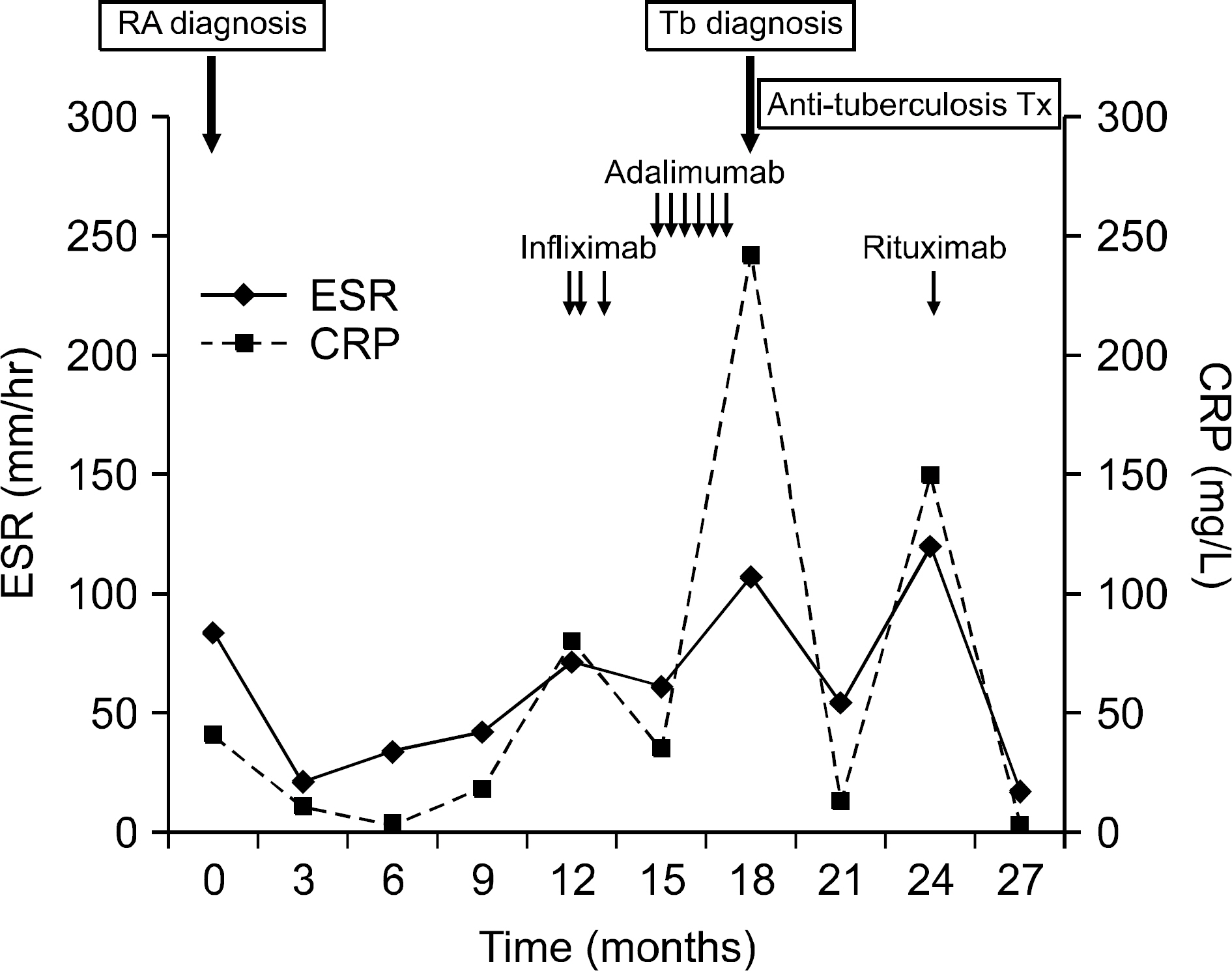
- One of the most important adverse effects of a tumor necrosis factor (TNF)-alpha inhibitor is the reactivation of tuberculosis. Most of them occur in the lung, but sometimes they can be found in other organs. Moreover, the proper management of active rheumatoid arthritis (RA) in patients with anti-TNF-alpha associated tuberculosis is still in debate. We present the case of a seropositive RA patient who showed good response with rituximab, an anti-CD20 monoclonal antibody, after developing splenic tuberuculosis, following treatment with TNF-alpha inhibitor. Confirming a diagnosis of splenic tuberculosis is difficult and can be delayed due to its nonspecific symptoms and rare occurrence. This case suggests that splenic tuberculosis should be doubted in RA patients treated with TNF-alpha inhibitor, and that rituximab may be considered as an alternative treatment option in RA patients with anti-TNF-alpha associated tuberculosis.
MeSH Terms
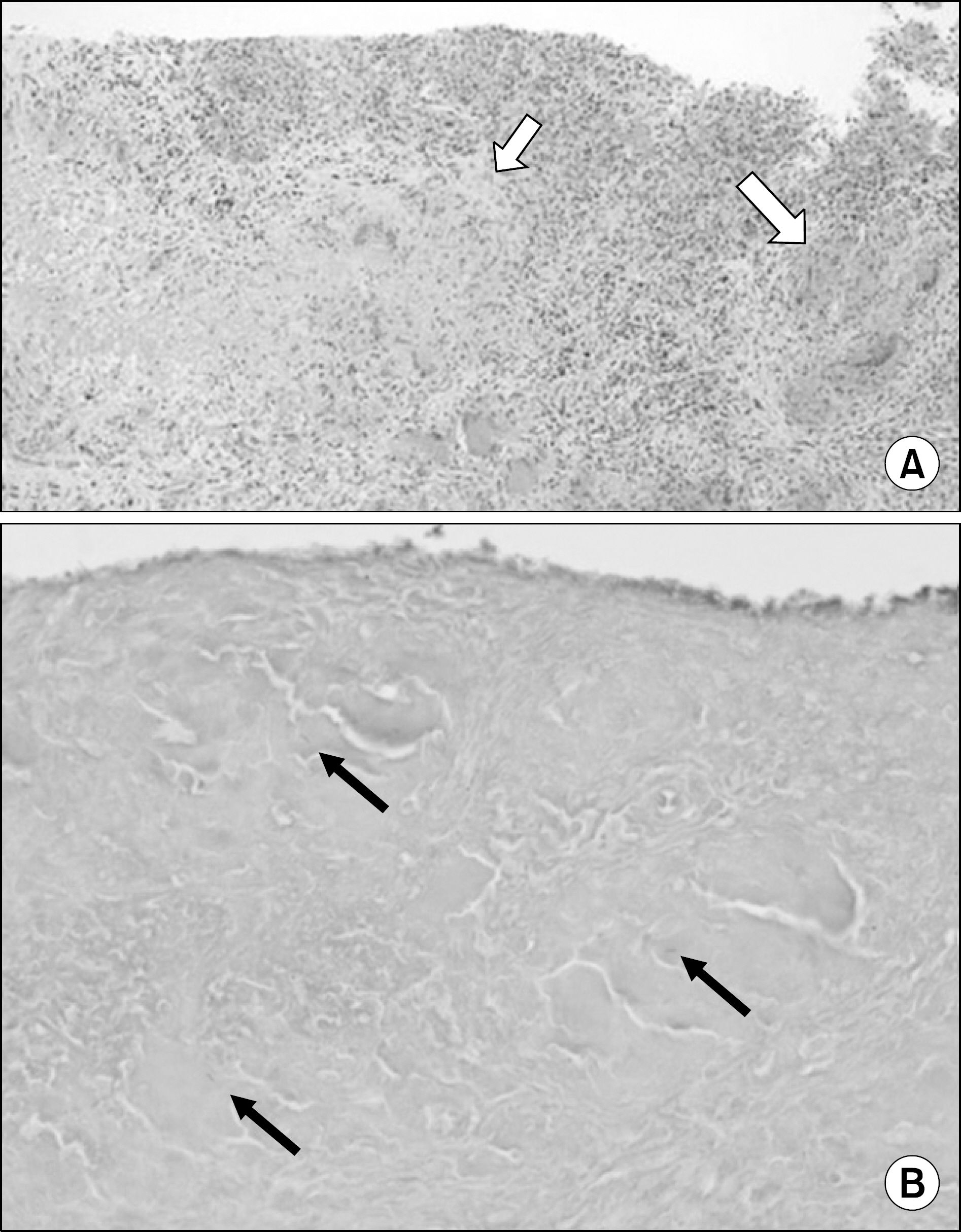
Figure
Reference
-
References
1. Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004; 350:2167–79.
Article2. Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006; 355:704–12.
Article3. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neu-tralizing agent. N Engl J Med. 2001; 345:1098–104.4. Gó mez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003; 48:2122–7.5. Samaila MO, Oluwole OP. Extrapulmonary tuberculosis: fine needle aspiration cytology diagnosis. Niger J Clin Pract. 2011; 14:297–9.
Article6. Fortaleza GT, Brito Mde F, Santos JB, Figueiredo AR, Gomes P. Splenic tuberculosis during psoriasis treatment with infliximab. An Bras Dermatol. 2009; 84:420–4.7. Cappello M, Randazzo C, Rizzuto G, Bonura C, Di Vita G, Galia M. Splenic tuberculosis in a patient with Crohn's disease on infliximab: case report. Inflamm Bowel Dis. 2010; 16:368–70.
Article8. Zhan F, Wang CJ, Lin JZ, Zhong PJ, Qiu WZ, Lin HH, et al. Isolated splenic tuberculosis: A case report. World J Gastrointest Pathophysiol. 2010; 1:109–11.
Article9. Yang Z, Kong Y, Wilson F, Foxman B, Fowler AH, Marrs CF, et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis. 2004; 38:199–205.
Article10. Burr ML, Malaviya AP, Gaston JH, Carmichael AJ, Ostör AJ. Rituximab in rheumatoid arthritis following an-ti-TNF-associated tuberculosis. Rheumatology (Oxford). 2008; 47:738–9.
Article11. Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999–2006. Thorax. 2009; 64:1090–5.
Article12. Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum. 2010; 39:327–46.
Article13. Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. REFLEX Trial Group. Rituximab for rheumatoid arthritis refractory to antitumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006; 54:2793–806.
Article14. Mohrbacher A. B cell non-Hodgkin's lymphoma: rituximab safety experience. Arthritis Res Ther. 2005; 7(Suppl 3):S19–25.15. Kang YA, Ko WJ, Kwon OJ, Kwon YS, Kim DH, Kim YK, et al. Korean guidelines for tuberculosis. 218. 2011.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Rituximab Use in Rheumatoid Arthritis Following Anti-TNF-Associated Tuberculosis
- Guidelines for Prevention of Tuberculosis in Patients with Rheumatoid Arthritis Treated with TNF-alpha Blockers
- New drugs for Rheumatoid arthritis
- A Case of Invasive Pulmonary Aspergillosis in a Patient with Rheumatoid Arthritis Treated with Adalimumab
- A Case of Tuberculous Arthritis Following the Use of Etanercept