Korean Diabetes J.
2009 Jun;33(3):206-214. 10.4093/kdj.2009.33.3.206.
Effect of Adipose Differentiation-Related Protein (ADRP) on Glucose Uptake of Skeletal Muscle
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. kspark@sun.ac.kr
- 2Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2222420
- DOI: http://doi.org/10.4093/kdj.2009.33.3.206
Abstract
- BACKGROUND
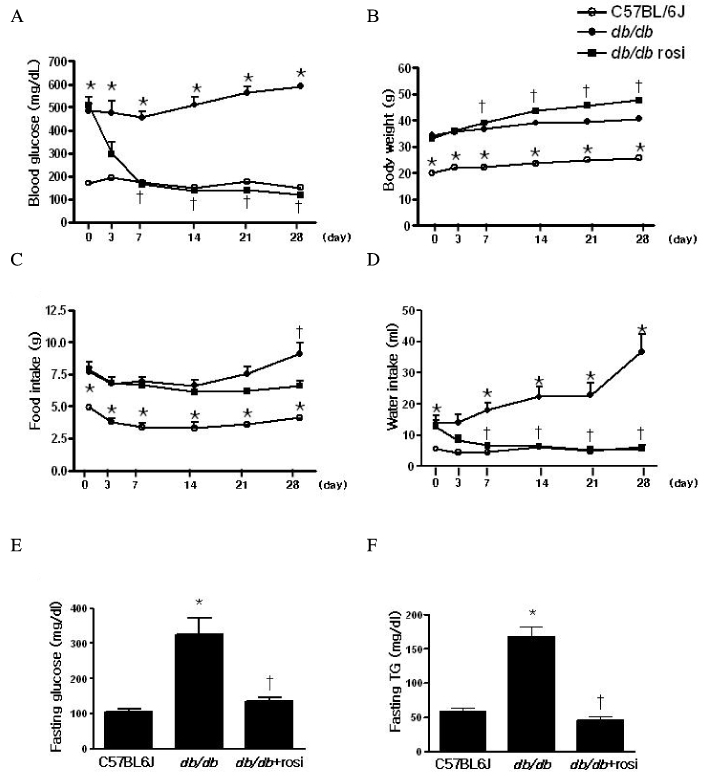
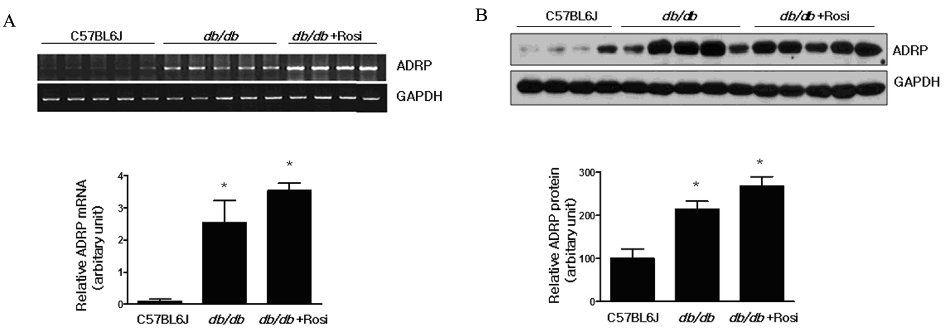
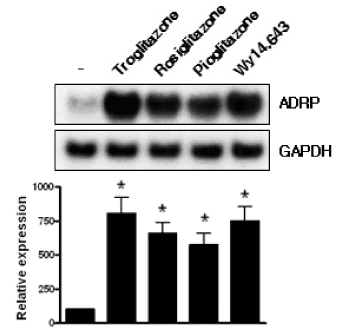
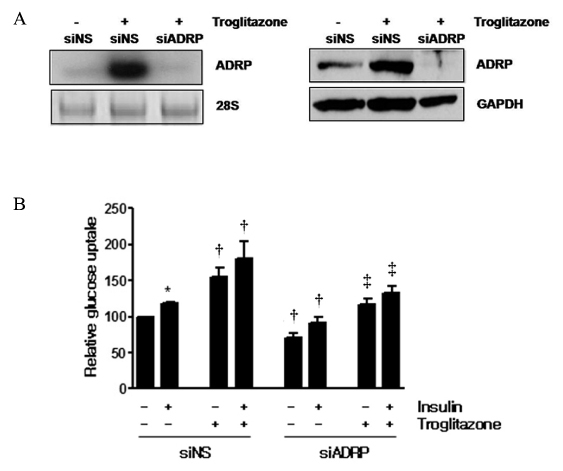
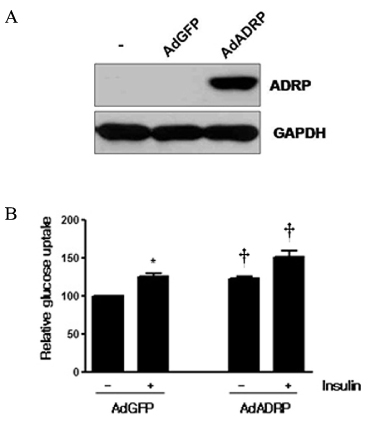
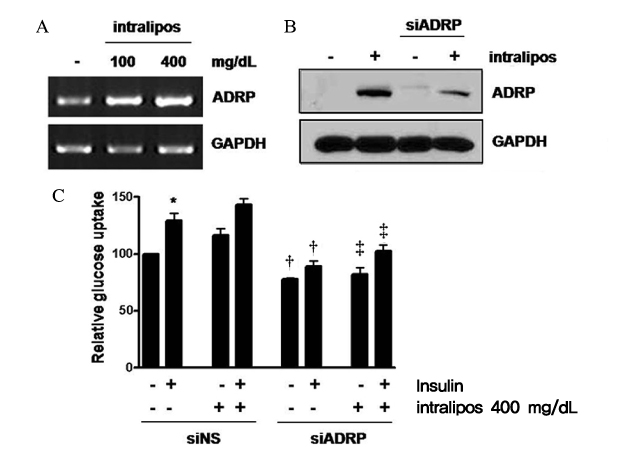
Skeletal muscle is the most important tissue contributing to insulin resistance. Several studies have shown that accumulation of intramyocellular lipid is associated with the development of insulin resistance. Thus, proteins involved in lipid transport, storage and metabolism might also be involved in insulin action in skeletal muscle. Adipose differentiation-related protein (ADRP), which is localized at the surface of lipid droplets, is known to be regulated by peroxisome proliferator activated receptor gamma (PPARgamma). However, it is not known whether ADRP plays a role in regulating glucose uptake and insulin action in skeletal muscle. METHODS: ADRP expression in skeletal muscle was measured by RT-PCR and western blot in db/db mice with and without PPARgamma agonist. The effect of PPARgamma agonist or high lipid concentration (0.4% intralipos) on ADRP expression was also obtained in cultured human skeletal muscle cells. Glucose uptake was measured when ADRP was down-regulated with siRNA or when ADRP was overexpressed with adenovirus. RESULTS: ADRP expression increased in the skeletal muscle of db/db mice in comparison with normal controls and tended to increase with the treatment of PPARgamma agonist. In cultured human skeletal muscle cells, the treatment of PPARgamma agonist or high lipid concentration increased ADRP expression. siADRP treatment decreased both basal and insulin-stimulated glucose uptake whereas ADRP overexpression increased glucose uptake in cultured human skeletal muscle cells. CONCLUSION: ADRP expression in skeletal muscle is increased by PPARgamma agonist or exposure to high lipid concentration. In these conditions, increased ADRP contributed to increase glucose uptake. These results suggest that insulin-sensitizing effects of PPARgamma are at least partially achieved by the increase of ADRP expression, and ADRP has a protective effect against intramyocellular lipid-induced insulin resistance.
Keyword
MeSH Terms
Figure
Reference
-
1. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004. 88:787–835.2. Bouzakri K, Koistinen HA, Zierath JR. Molecular Mechanisms of Skeletal Muscle Insulin Resistance in Type 2 Diabetes. Curr Diabetes Rev. 20. 1:167–174.3. Krssak M, FalkPetersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999. 42:113–116.4. Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996. 45:947–950.5. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997. 46:983–988.6. Lu X, Gruia-Gray J, Copeland NG, Gilbert DJ, Jenkins NA, Londos C, Kimmel AR. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome. 2001. 12:741–749.7. Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997. 38:2249–2263.8. Shaw CS, Sherlock M, Stewart PM, Wagenmakers AJ. Adipophilin distribution and colocalisation with lipid droplets in skeletal muscle. Histochem Cell Biol. 2009. 131:575–581.9. Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998. 294:309–321.10. Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008. 57:2037–2045.11. Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res. 2007. 48:2751–2761.12. Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005. 288:E1195–E1205.13. Targett-Adams P, McElwee MJ, Ehrenborg E, Gustafsson MC, Palmer CN, McLauchlan J. A PPAR response element regulates transcription of the gene for human adipose differentiation-related protein. Biochim Biophys Acta. 2005. 1728:95–104.14. Motomura W, Inoue M, Ohtake T, Takahashi N, Nagamine M, Tanno S, Kohgo Y, Okumura T. Up-regulation of ADRP in fatty liver in human and liver steatosis in mice fed with high fat diet. Biochem Biophys Res Commun. 2006. 340:1111–1118.15. Mak KM, Ren C, Ponomarenko A, Cao Q, Lieber CS. Adipose differentiation-related protein is a reliable lipid droplet marker in alcoholic fatty liver of rats. Alcohol Clin Exp Res. 2008. 32:683–689.16. Edvardsson U, Ljungberg A, Linden D, William-Olsson L, Peilot-Sjogren H, Ahnmark A, Oscarsson J. PPARalpha activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes. J Lipid Res. 2006. 47:329–340.17. Dalen KT, Ulven SM, Arntsen BM, Solaas K, Nebb HI. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J Lipid Res. 2006. 47:931–943.18. Chawla A, Lee CH, Barak Y, He W, Rosenfeld J, Liao D, Han J, Kang H, Evans RM. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci U S A. 2003. 100:1268–1273.19. Jun Gao, Hong Ye, Ginette Serrero. Stimulation of adipose differentiation related protein (ADRP) expression in adipocyte precursors by long-chain fatty acids. J Cell Physiol. 2000. 182:297–302.20. Phillips SA, Choe CC, Ciaraldi TP, Greenberg AS, Kong AP, Baxi SC, Christiansen L, Mudaliar SR, Henry RR. Adipocyte differentiation-related protein in human skeletal muscle: relationship to insulin sensitivity. Obes Res. 2005. 13:1321–1329.21. Magnusson Björn, Asp Lennart, Boström Pontus, Ruiz Michel, Stillemark-Billton Pia, Lindén Daniel, Borén Jan, Olofsson Sven-Olof. Adipocyte Differentiation-Related Protein Promotes Fatty Acid Storage in Cytosolic Triglycerides and Inhibits Secretion of Very Low-Density Lipoproteins. Arterioscler Thromb Vasc Biol. 2006. 26:1566–1571.22. Jiang HP, Serrero G. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proc Natl Acad Sci USA. 1992. 89:7856–7860.23. Jenkins AB, Storlien LH, Chisholm DJ, Kraegen EW. Effects of nonesterified fatty acid availability on tissue-specific glucose utilization in rats in vivo. J Clin Invest. 1988. 82:293–299.24. Hirabara SM, Silveira LR, Abdulkader F, Carvalho CR, Procopio J, Curi R. Time-dependent effects of fatty acids on skeletal muscle metabolism. J Cell Physiol. 2007. 210:7–15.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of exercise-stimulated glucose uptake in skeletal muscle
- Effects of insulin and exercise on glucose uptake of skeletal muscle in diabetic rats
- Effect of Age on Glucose Metabolism of Skeletal Muscle in Rats
- Type 2 Diabetes Mellitus and Retinol-Binding Protein 4
- Roles of Protein Arginine Methyltransferases in the Control of Glucose Metabolism