J Korean Ophthalmol Soc.
2013 Nov;54(11):1641-1648. 10.3341/jkos.2013.54.11.1641.
Density of Orbital Fat and Extraocular Muscle in Thyroid-Associated Myopathy and Idiopathic Orbital Myositis
- Affiliations
-
- 1Department of Ophthalmology, Dong-A University College of Medicine, Busan, Korea. hbahn@dau.ac.kr
- KMID: 2217908
- DOI: http://doi.org/10.3341/jkos.2013.54.11.1641
Abstract
- PURPOSE
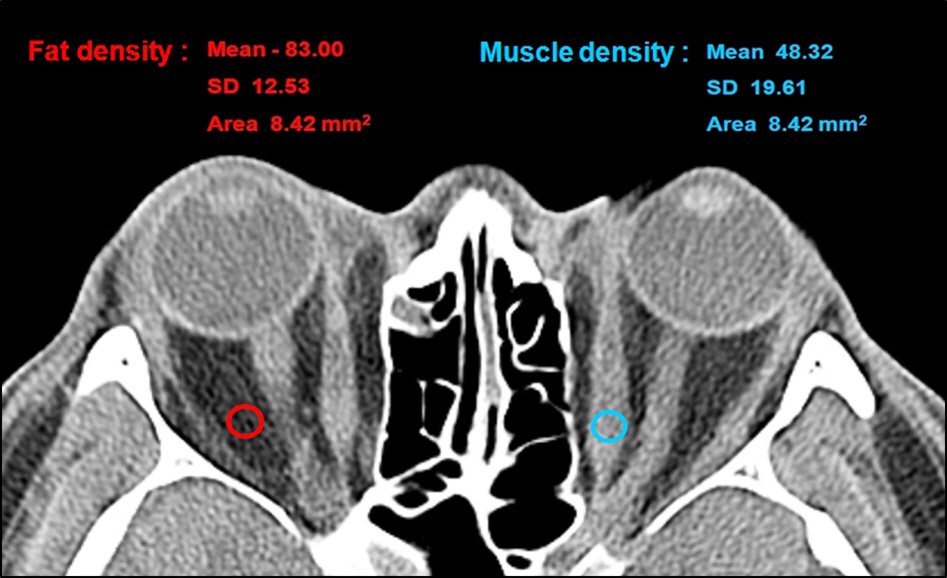
To perform and compare differential diagnosis of patients with thyroid-associated myopathy, idiopathic orbital myositis and normal controls based on orbital computed tomography. Orbital fat and extraocular muscle densities were quantified using Hounsfield Unit (HU) and their characteristics were compared and analyzed.
METHODS
From February 2005 to January 2013, orbital computed tomography was performed on 90 eyes of 47 thyroid-associated myopathy patients, 18 eyes of 14 idiopathic orbital myositis patients and 280 eyes of 140 normal subjects. The average values of orbital fat and extraocular muscle densities were measured and compared using HU. The density differences between the patients with thyroid-associated myopathy and the normal group were analyzed by age, clinical activity score, ocular protrusion and disease duration.
RESULTS
In the thyroid-associated myopathy group, orbital fat and extraocular muscle densities were -87.8 +/- 12.5 HU and 48.7 +/- 7.1 HU, respectively. In the idiopathic orbital myositis group, the orbital fat and extraocular muscle densities were 79.9 +/- 9.9 HU and 49.2 +/- 9.1 HU, respectively. There was a statistically significant lower result of orbital fat in the thyroid-associated myopathy group (p = 0.002), however, the extraocular muscle density did not show a statistically significant difference (p = 0.775). The orbital fat and extraocular muscle densities of the normal group were -79.0 +/- 11.2 HU and 54.3 +/- 6.3 HU, respectively. There were significantly lower results in both orbital fat and extraocular muscle densities in the thyroid-associated myopathy group than normal group (p = 0.000). In active cases and those accompanied by ocular protrusion, there was no significant difference in orbital fat density (p = 0.345 and p = 0.952, respectively), while extraocular muscle density significantly decreased (p = 0.007 and p = 0.003, respectively).
CONCLUSIONS
A difference between the orbital fat and extraocular muscle densities in thyroid-associated myopathy and idiopathic orbital myositis could be quantitatively found using HU and orbital computed tomography.
Keyword
Figure
Reference
-
References
1. Lutt JR, Lim LL, Phal PM, Rosenbaum JT. Orbital inflammatory disease. Semin Arthritis Rheum. 2008; 37:207–22.
Article2. Tanenbaum M, McCord Jr CD, Nunery WR. Grave's Ophthalmopathy. McCord CD, Tanenbaum M, Nunery WR, editors. Oculoplastic Surgery. 3rd ed.New York, NY: Raven Press, Ltd;1995:p. 379–416.3. Kiljanski JI, Nebes V, Wall JR. The ocular muscle cell is a target of the immune system in endocrine ophthalmopathy. Int Arch Allergy Immunol. 1995; 106:204–12.
Article4. Bahn RS, Heufelder AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med. 1993; 329:1468–75.
Article5. Nunery WR. Ophthalmic Graves’ disease: A dual theory of pathogenesis. Ophthalmol Clin North Am. 1991; 4:73–87.6. Kim JR, Yim HB, Chung SK. Risk factors for dry eye in thy- roid-associated ophthalmopathy. J Korean Ophthalmol Soc. 2011; 52:771–6.7. Bartley GB, Fatourechi V, Kadrmas EF, et al. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996; 121:284–90.
Article8. Mombaerts I, Koornneef L. Current status in the treatment of orbi- tal myositis. Ophthalmology. 1997; 104:402–8.9. Mannor GE, Rose GE, Moseley IF, Wright JE. Outcome of orbital myositis. Clinical features associated with recurrence. Ophthalmology. 1997; 104(409):13. discussion, 414.10. Scott IU, Siatkowski RM. Idiopathic orbital myositis. Curr Opin Rheumatol. 1997; 9:504–12.
Article11. Nugent RA, Belkin RI, Neigel JM, et al. Graves orbitopathy: Correlation of CT and clinical findings. Radiology. 1990; 177:675–82.
Article12. Kim DH, Kim SH, Oh JH. The measurement of size of human ex- traocular muscles and their changes in thyroid associated oph- thalmopathy in Korea. J Korean Ophthalmol Soc. 2001; 42:321–8.13. Regensburg NI, Wiersinga WM, Berendschot TT, et al. Densities of orbital fat and extraocular muscles in Graves’ orbitopathy pa- tients and controls. Ophthal Plast Reconstr Surg. 2011; 27:236–40.14. Gerding MN, van der Meer JW, Broenink M, et al. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol (Oxf). 2000; 52:267–71.
Article15. Werner SC. Modification of the classification of the eye changes of Graves’ disease: Recommendations of the Ad Hoc Committee of the American thyroid association. J Clin Endocrinol Metab. 1977; 44:203–4.
Article16. Eckstein AK, Plicht M, Lax H, et al. Thyrotropin receptor autoanti- bodies are independent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006; 91:3464–70.17. Dijkstal JM, Bothun ED, Harrison AR, Lee MS. Normal exoph- thalmometry measurements in United states pediatric population. Ophthal Plast Reconstr Surg. 2012; 28:54–6.18. Yang YW, Kim OY. A clinical analysis of thyroid diseases among Korean. J Korean Surg Soc. 1994; 46:781–94.19. Choi KM, Kim OY. Thyroid diseases in Korean. J Korean Surg Soc. 1991; 40:13–9.20. Dolman PJ, Rootman J. VISA Classification for Graves’ orbitopathy. Ophthal Plast Reconstr Surg. 2006; 22:319–24.
Article21. Kendler DL, Lippa J, Rootman J. The initial clinical characteristics of Graves’ orbitopathy vary with age and sex. Arch Ophthalmol. 1993; 111:197–201.
Article22. Jang SY, Lee SY, Lee EJ, Yoon JS. Clinical features of thyroid as- sociated ophthalmopathy in clinically euthyroid Korean patients. Eye (Lond). 2012; 26:1263–9.23. Forbes GS, Earnest F 4th, Waller RR. Computed tomography of or- bital tumors, including late-generation scanning techniques. Radiology. 1982; 142:387–94.24. Feldon SE, Lee CP, Muramatsu SK, Weiner JM. Quantitative com- puted tomography of Graves’ ophthalmopathy. Extraocular muscle and orbital fat in development of optic neuropathy. Arch Ophthalmol. 1985; 103:213–5.25. Hallin ES, Feldon SE. Graves’ ophthalmopathy: I. simple CT esti- mates of extraocular muscle volume. Br J Ophthalmol. 1988; 72:674–7.26. Chen YL, Chang TC, Huang KM, et al. Relationship of eye move- ment to computed tomographic findings in patients with Graves’ ophthalmopathy. Acta Ophthalmol (Acta Ophthalmol). 1994; 72:472–7.27. Park JM, Ahn HB, Lee JH. The clinical features and the change of extraocular muscle at the first visit in hyperthyroidism patients. J Korean Ophthalmol Soc. 2003; 44:2197–203.28. Mull RT. Mass estimates by computed tomography: Physical den- sity from CT numbers. AJR Am J Roentgenol. 1984; 143:1101–4.29. Kim DJ, Lee SI, Song DW, Cheong TY. Relations between ex- tracorporeal shockwave lithotripsy success and hounsfield units. Kwandong Med J. 2006; 10:13–5.30. Choi HJ, Lee HJ, Kang SG. The clinical significance of hounsfield number of metallic and non-metallic foreign bodies in the soft tissue. Soonchunhyang Med J. 2010; 16:226–30.31. Kim HC, Cho JH. Differentiation of chromophobe renal cell carci- noma and clear cell renal cell carcinoma by using helical CT. Yeungnam Univ J Med. 2012; 29:14–8.32. Garrity JA, Bahn RS. Pathogenesis of graves ophthalmopathy: Implications for prediction, prevention, and treatment. Am J Ophthalmol. 2006; 142:147–53.
Article33. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010; 362:726–38.
Article34. Bartalena L, Lai A, Compri E, et al. Novel immunomodulating agents for Graves’ orbitopathy. Ophthal Plast Reconstr Surg. 2008; 24:251–6.
Article35. Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003; 24:802–35.
Article36. Smith TJ, Bahn RS, Gorman CA, Cheavens M. Stimulation of gly- cosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991; 72:1169–71.37. Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol. 1995; 268(2 Pt 1):C382–8.
Article38. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004; 89:5076–80.
Article39. Kim DH, Kim SJ, Oh JH. The measurement of size of human extra- ocular muscles and their changes in thyroid associated ophthalm- opathy in Korea. J Korean Ophthalmol Soc. 2001; 42:321–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Idiopathic Orbital Myositis Involving All Extraocular Muscles of Both Eyes
- Effect of Corticosteroid on Orbital Pseudotumor Caused by Orbital Myositis
- Isolated Unilateral Ptosis Caused by Idiopathic Orbital Myositis
- Unilateral Ptosis Due to Isolated Levator Myositis
- A Case of Idiopathic Orbital Myositis