J Korean Ophthalmol Soc.
2013 Apr;54(4):618-626. 10.3341/jkos.2013.54.4.618.
Analysis of Intraocular Cytokines According to Progression of Diabetic Retinopathy and Macular Edema in Diabetic Patients
- Affiliations
-
- 1Department of Ophthalmology, Hallym University College of Medicine, Seoul, Korea. sungpyo@hanafos.com
- 2Department of Laboratory Medicine, Hallym University College of Medicine, Seoul, Korea.
- KMID: 2216943
- DOI: http://doi.org/10.3341/jkos.2013.54.4.618
Abstract
- PURPOSE
To investigate the relationship between the aqueous concentrations of cytokines, chemokine, VEGF and the severity of diabetic retinopathy and diabetic macular edema.
METHODS
Sixty eyes with diabetic retinopathy and diabetic macular edema, and 10 eyes of nondiabetic subjects were followed for 6 months. The aqueous levels of inflammatory and anti-inflammatory factors were measured. The aqueous levels of cytokines were investigated according to the severity of diabetic retinopathy and diabetic macular edema.
RESULTS
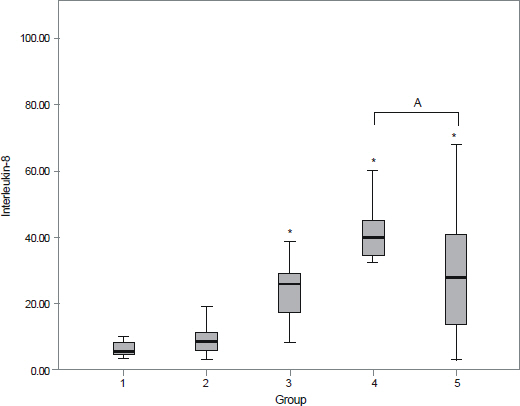
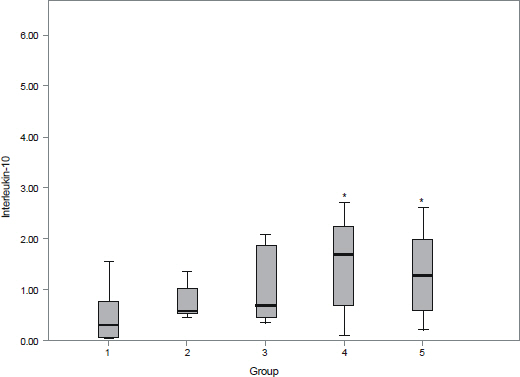
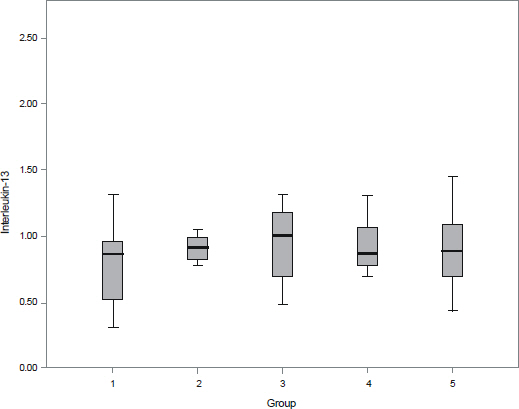
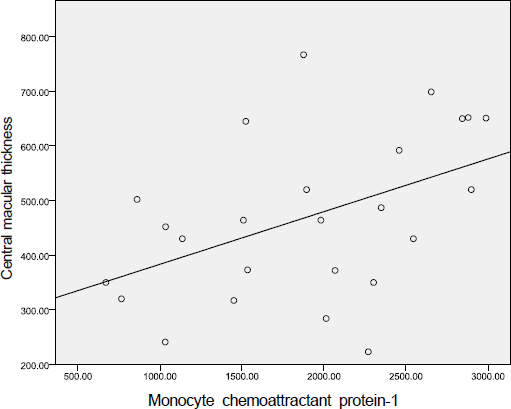
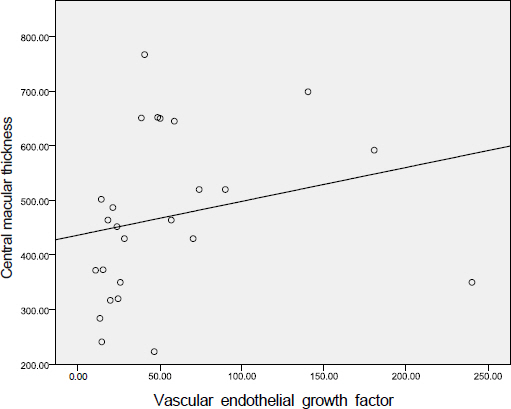
The aqueous levels of IL-6, IL-8, IL-10, MCP-1, and VEGF were elevated according to the severity of diabetic retinopathy. The aqueous levels of IL-13 were higher in eyes of diabetic patients than in eyes of nondiabetic subjects, but there were no statistically significant difference between diabetic retinopathy and nondiabetic subjects. The aqueous levels of IL-6, MCP-1, and VEGF were positively correlated with central macular thickness in eyes with diabetic macular edema.
CONCLUSIONS
The aqueous levels of IL-6, IL-8, IL-10, MCP-1, and VEGF were correlated with the severity of diabetic retinopathy. The aqueous levels of IL-6, MCP-1, and VEGF were positively correlated with the severity of macular edema.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Analysis of Aqueous Humor Cytokines in Diabetic Retinopathy
Young Jin Song, Jong Hyun Jung, Do Gyun Kim
J Korean Ophthalmol Soc. 2014;55(12):1821-1827. doi: 10.3341/jkos.2014.55.12.1821.Elevated Matrix Metalloproteinase in Aqueous Humor in Patients with Open-Angle Glaucoma
Hyo Won Kim, Dong Hyun Jee, Jin A Choi
J Korean Ophthalmol Soc. 2016;57(4):601-606. doi: 10.3341/jkos.2016.57.4.601.
Reference
-
References
1. Murugeswari P, Shukla D, Rajendran A, et al. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and Eales' disease. Retina. 2008; 28:817–24.
Article2. Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular enthothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002; 133:70–7.3. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–7.
Article4. Boyd SR, Zachary I, Chakravarthy U, et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Arch Ophthalmol. 2002; 120:1644–50.
Article5. Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology. 1994; 101:1061–70.
Article6. Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in diabetic population. Ophthalmology. 1998; 105:998–1003.7. Funatsu H, Yamashita H, Ikeda T, et al. Vitreous levels of inter-leukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003; 110:1690–6.
Article8. Funatsu H, Noma H, Mimura T, et al. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009; 116:73–9.
Article9. Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004; 18:1450–2.
Article10. Paccola L, Costa RA, Folgosa MS, et al. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular edema(IBEME study). Br J Ophthalmol. 2008; 92:76–80.11. Early treatment Diabetic Retinopathy Study Research group. Grading diabetic retinopathy from stereoscopic color fundus photographs-An extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991; 98:786–806.12. Fisman EZ, Motro M, Tenenbaum A. Cardiovascular diabetology in the core of a novel interleukins classification: the bad, the good and the aloof. Cardiovasc Diabetol. 2003; 2:11.13. Elshal MF, McCoy JP. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods. 2006; 38:317–23.
Article14. Dupont NC, Wang K, Wadhwa PD, et al. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: Determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005; 66:175–91.
Article15. Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009; 94:3171–82.
Article16. Gustavsson C, Agardh CD, Agardh E. Profile of intraocular tumour necrosis factor-α and interleukin-6 in diabetic subjects with different degrees of diabetic retinopathy. Acta Ophthalmol. 2012; doi: 10.1111/j.1755-3768.2012.02430.
Article17. Hoekzema R, Murray PI, van Haren MA, et al. Analysis of inter-leukin-6 in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1991; 32:88–95.18. Hernandez C, Segura R, Fonollosa A, et al. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005; 22:719–22.
Article19. Kim KS, Rajagopal V, Gonsalves C, et al. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxiamediated expression of IL-8 chemokine in human endothelial cells. J Immunol. 2006; 177:7211–24.
Article20. Schober A, Zernecke A. Chemokines in vascular remodeling. Thromb Haemost. 2007; 97:730–7.
Article21. Tashimo A, Mitamura Y, Nagai S, et al. Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabet Med. 2004; 21:1292–7.
Article22. Myśliwiec M, Zorena K, Balcerska A, et al. The activity of N-acetyl-beta-D-glucosaminidase and tumor necrosis factor-alpha at early stage of diabetic retinopathy development in type 1 diabetes mellitus children. Clin Biochem. 2006; 39:851–6.
Article23. Silvestre JS, Mallat Z, Duriez M, et al. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ Res. 2000; 87:448–52.
Article24. Cheung CM, Vania M, Ang M, et al. Comparison of aqueous humor cytokine and chemokine levels in diabetic patients with and without retinopathy. Mol Vis. 2012; 18:830–7.25. Ozturk BT, Bozkurt B, Kerimoglu H, et al. Effect of serum cytokines and VEGF levels on diabetic retinopathy and macular thickness. Mol Vis. 2009; 15:1906–14.26. Rangasamy S, McGuire PG, Das A. Diabetic retinopathy and inflammation: novel therapeutic targets. Middle East Afr J Ophthalmol. 2012; 19:52–9.
Article27. Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003; 111:677–90.
Article28. Chen H, Wen F, Zhang X, Su SB. Expression of T-helper-associated cytokines in patients with type 2 diabetes mellitus with retinopathy. Mol Vis. 2012; 18:219–26.29. Hanifi-Moghaddam P, Kappler S, Seissler J, et al. Altered chemokine levels in individuals at risk of Type 1 diabetes mellitus. Diabet Med. 2006; 23:156–63.
Article30. Roberge FG, de Smet MD, Benichou J, et al. Treatment of uveitis with recombinant human interleukin-13. Br J Ophthalmol. 1998; 82:1195–8.
Article31. Haas CS, Amin MA, Ruth JH, et al. In vivo inhibition of angio-genesis by interleukin-13 gene therapy in a rat model of rheumatoid arthritis. Arthritis Rheum. 2007; 56:2535–48.
Article32. Fisman EZ, Motro M, Tenenbaum A. Cardiovascular diabetology in the core of a novel interleukins classification: the bad, the good and the aloof. Cardiovasc Diabetol. 2003; 2:11.33. Noma H, Funatsu H, Yamasaki M, et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond). 2008; 22:42–8.
Article34. Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005; 243:3–8.
Article35. Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007; 114:855–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Evaluation about the Impact of Cataract Operation on the Progression of Diabetic Retinopathy
- Comparison of the Clinical Diagnosis of Diabetic Macular Edema with Diagnosis by Retinal Thickness Analyzer
- Clinical Analysis of Combined Vitrectomy and Phacoemulsification with Intraocular Lens Implantation for Proliferative Diabetic Retinopathy
- A Case of Rapid Progression to Proliferative Diabetic Retinopathy Associated with Generalized Edema
- Grid-Pattern Laser Photocoagulation in Diabetic Macular Edema