J Korean Ophthalmol Soc.
2011 Jan;52(1):86-92. 10.3341/jkos.2011.52.1.86.
The Study of Characteristics of Acellular Porcine Cornea Using Freezing-Thawing-Centrifugation
- Affiliations
-
- 1Department of Ophthalmology, Chungang University Yongsan Hospital, Seoul, Korea. jck50ey@kornet.net
- KMID: 2214142
- DOI: http://doi.org/10.3341/jkos.2011.52.1.86
Abstract
- PURPOSE
To develop a new decellularization technique of porcine cornea using freezing-thawing-centrifugation (FTC) and to examine the characteristics of acellular porcine cornea (APC) for xenograft material.
METHODS
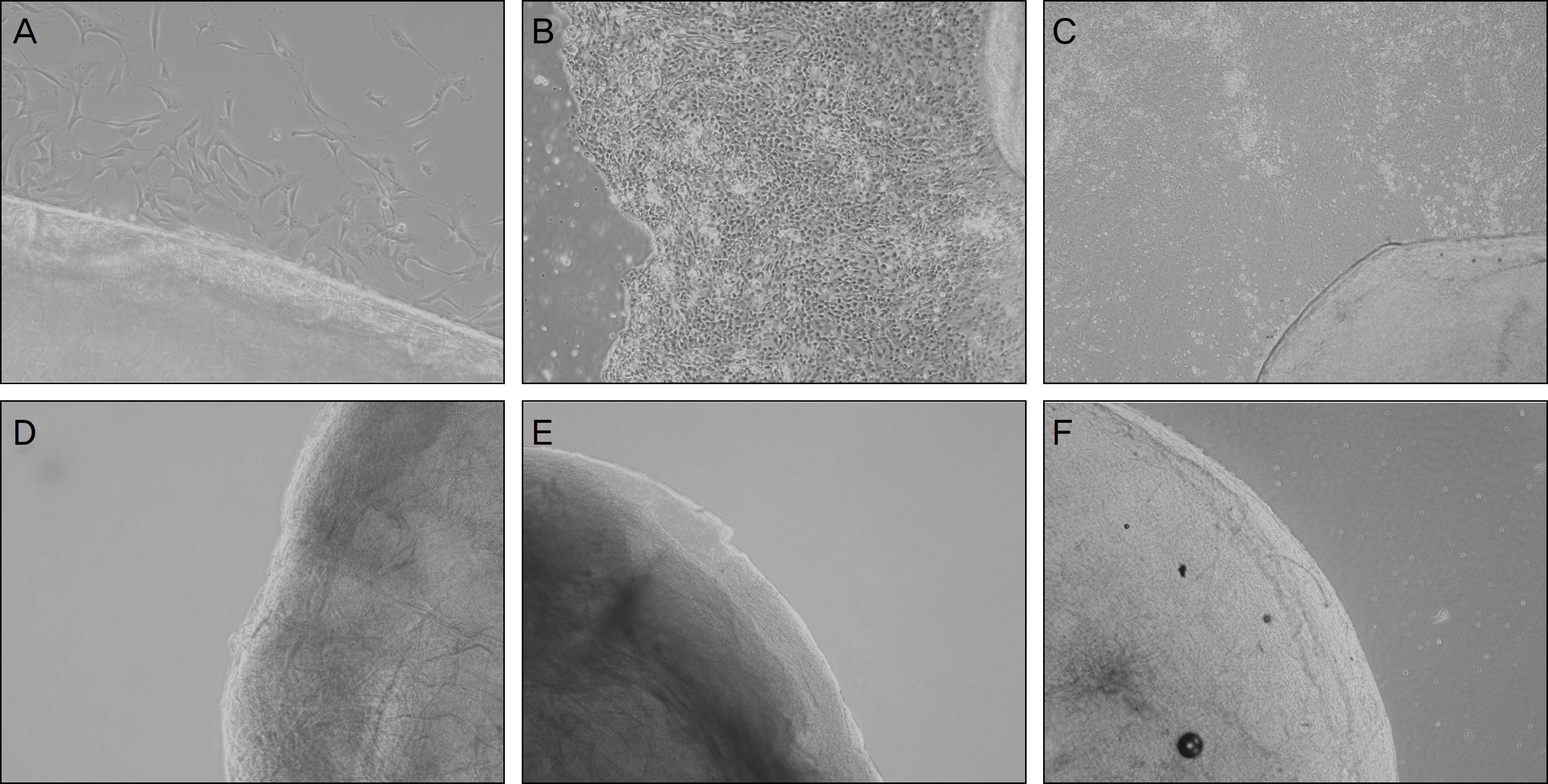
Two-hundred micrometer thickness porcine corneas were decellularized with DNase/RNase, followed by 3 freezing-thawing-centrifugations (FTC, group 1), lyophilized FTC-APC (group 2), and chemical enzyme treated APC (CE-APC, group 3). Histologic evaluation to examine cells and collagen matrix, comparison of transparency, and cultivation to determine the viability of stromal cells was performed in fresh porcine cornea and 3 experimental groups.
RESULTS
Decellularization occurred successfully in all experimental groups. Decellularization was confirmed by H&E staining and cultivation. Transparency of group 1 was similar to the normal porcine cornea but transparency of group 2 and group 3 was decreased. Collagen fibers of CE-APC (group 3) were not as well arrayed as FTC-APC (group 2).
CONCLUSIONS
Acellularity of porcine cornea was successfully achieved by the FTC method with preservation of the cornea stroma. Novel decellularized porcine cornea can be considered as xenogeneic material for corneal transplantation.
Keyword
Figure
Reference
-
References
1. Sedlakova K, Filipec M. Effect of suturing technique on corneal xenograft survival. Cornea. 2007; 26:1111–4.
Article2. Lee JK, Ryu YH, Ahn JI, et al. The effect of lyophilization on graft acceptance in experimental xenotransplantation using porcine cornea. Artif Organs. 2010; 34:37–45.
Article3. Choi SH, Lee YW, Kim HM, et al. Epidemiologic studies of keratoplasty in Korea. J Korean Ophthalmol Soc. 2006; 47:538–47.4. Melles GR, Remeijer L, Geerards AJ, Beekhuis WH. A quick surgical technique for deep anterior lamellar keratoplasty using viscodissection. Cornea. 2000; 19:427–32.
Article5. Lin XC, Hui YN, Wang YS, et al. Lamellar keratoplasty with a graft of lyophilized acellular porcine corneal stroma in the rabbit. Vet Ophthalmol. 2008; 11:61–6.
Article6. Amano S, Shimomura N, Kaji Y, et al. Antigenicity of porcine cornea as xenograft. Curr Eye Res. 2003; 26:313–8.
Article7. Xu YG, Xu YS, Huang C, et al. Development of a rabbit corneal equivalent using an acellular corneal matrix of a porcine substrate. Mol Vis. 2008; 14:2180–9.8. Lee HI, Kim MK, Ko JH, et al. The Characteristics of Porcine Cornea as a Xenograft. J Korean Ophthalmol Soc. 2006; 47:2020–9.9. Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006; 27:3675–83.
Article10. López-García JS, Rivas Jara L, García-Lozano I, et al. aberrations limbus evolution after alkaline burns. Cornea. 2007; 26:1043–8.11. Kim MS, Lee SC, Lee SJ, JIN KH. Predictability of Donor Lamellar Graft Thickness and Diameter Using a Microkeratome in Porcine Eyes. J Korean Ophthalmol Soc. 2007; 48:473–7.12. D'Amico RA, Chaunico R, Castroviejo R. Suppression of the immune response to corneal xenografts. Transplant Proc. 1969; 1:256–8.13. Auchincloss H Jr. Xenogeneic transplantation. Transplantation. 1988; 46:1–20.
Article14. Insler MS, Lopez JG. Heterologous transplantation versus enhancement of human corneal endothelium. Cornea. 1991; 10:136–48.
Article15. Ross JR, Howell DN, Sanfilippo FP. Characteristics of corneal xenograft rejection in a discordant species combination. Invest Ophthalmol Vis Sci. 1993; 34:2469–76.16. Li C, Xu JT, Kong FS, Li JL. Experimental studies on penetrating heterokeratoplasty with human corneal grafts in monkey eyes. Cornea. 1992; 11:66–72.
Article17. Tseng YL, Kuwaki K, Dor FJ, et al. Alpha1, 3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005; 80:1493–500.18. Rosenbluth J, Schiff R, Liang WL, et al. Xenotransplantation of transgenic oligodendrocyte-lineage cells into spinal cord-injured adult rats. Exp Neurol. 1997; 147:172–82.
Article19. Tanaka K, Yamada J, Streilein JW. Xenoreactive CD4+ T cells and acute rejection of orthotopic guinea pig corneas in mice. Invest Ophthalmol Vis Sci. 2000; 41:1827–32.20. Freytes DO, Badylak SF, Webster TJ, et al. Biaxial strength of mul-tilaminated extracellular matrix scaffolds. Biomaterials. 2004; 25:2353–61.
Article21. De Filippo RE, Yoo JJ, Atala A. Urethral replacement using cell seeded tubularized collagen matrices. J Urol. 2002; 168:1789–92.
Article22. Jackson DW, Grood ES, Cohn BT, et al. The effects of in situ freezing on the anterior cruciate ligament. An experimental study in goats. J Bone Joint Surg Am. 1991; 73:201–13.
Article23. Gulati AK. Evaluation of acellular and cellular nerve grafts in repair of rat peripheral nerve. J Neurosurg. 1988; 68:117–23.
Article24. Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008; 372:2023–30.
Article25. Freytes DO, Badylak SF, Webster TJ, et al. Biaxial strength of mul-tilaminated extracellular matrix scaffolds. Biomaterials. 2004; 25:2353–61.
Article26. Lin P, Chan WC, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004; 10:1046–53.
Article27. Vyavahare N, Hirsch D, Lerner E, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms. Circulation. 1997; 95:479–88.28. Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003; 12:659–66.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Freezing Method, Thawing Temperature and Post-thaw Dilution/Washing on Motility and Morphology Characteristics of High-quality Human Sperm
- A study on the changes of sperm motility according to freezing and thawing methods
- Freezing and Thawing Conditions of Rat Hepatocytes
- A Case of Tectonic Lamellar Corneal Patch Graft Using Acellular Cornea in Corneal Ulcer Perforation
- Comparison of Vitrification and Slow Freezing for the Cryopreservation of Mouse Pronuclear Stage Embryos