J Korean Ophthalmol Soc.
2009 Jul;50(7):989-995. 10.3341/jkos.2009.50.7.989.
The Effects of Amniotic Membrane Contact Lens for Cornea Wound Healing
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Dong-A University, Busan, Korea. wcpark@daunet.donga.ac.kr
- KMID: 2212485
- DOI: http://doi.org/10.3341/jkos.2009.50.7.989
Abstract
- PURPOSE
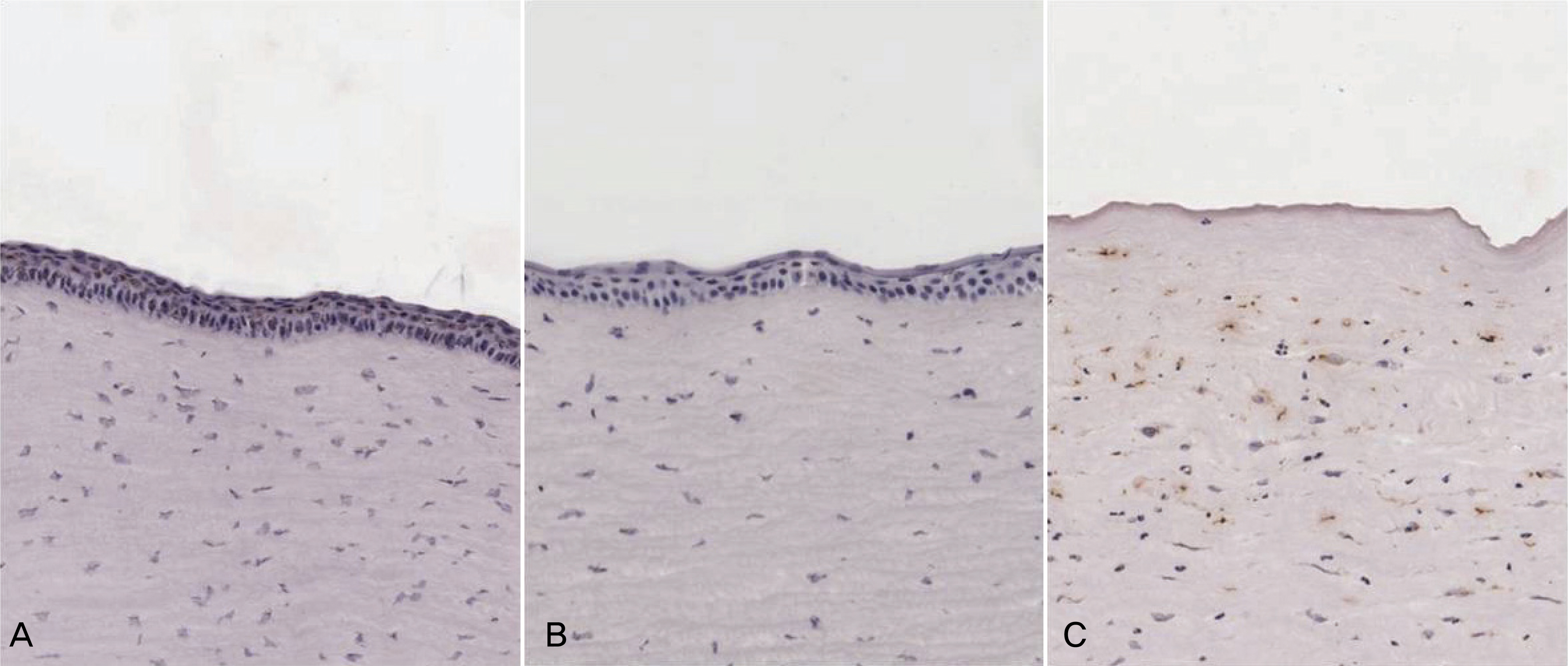
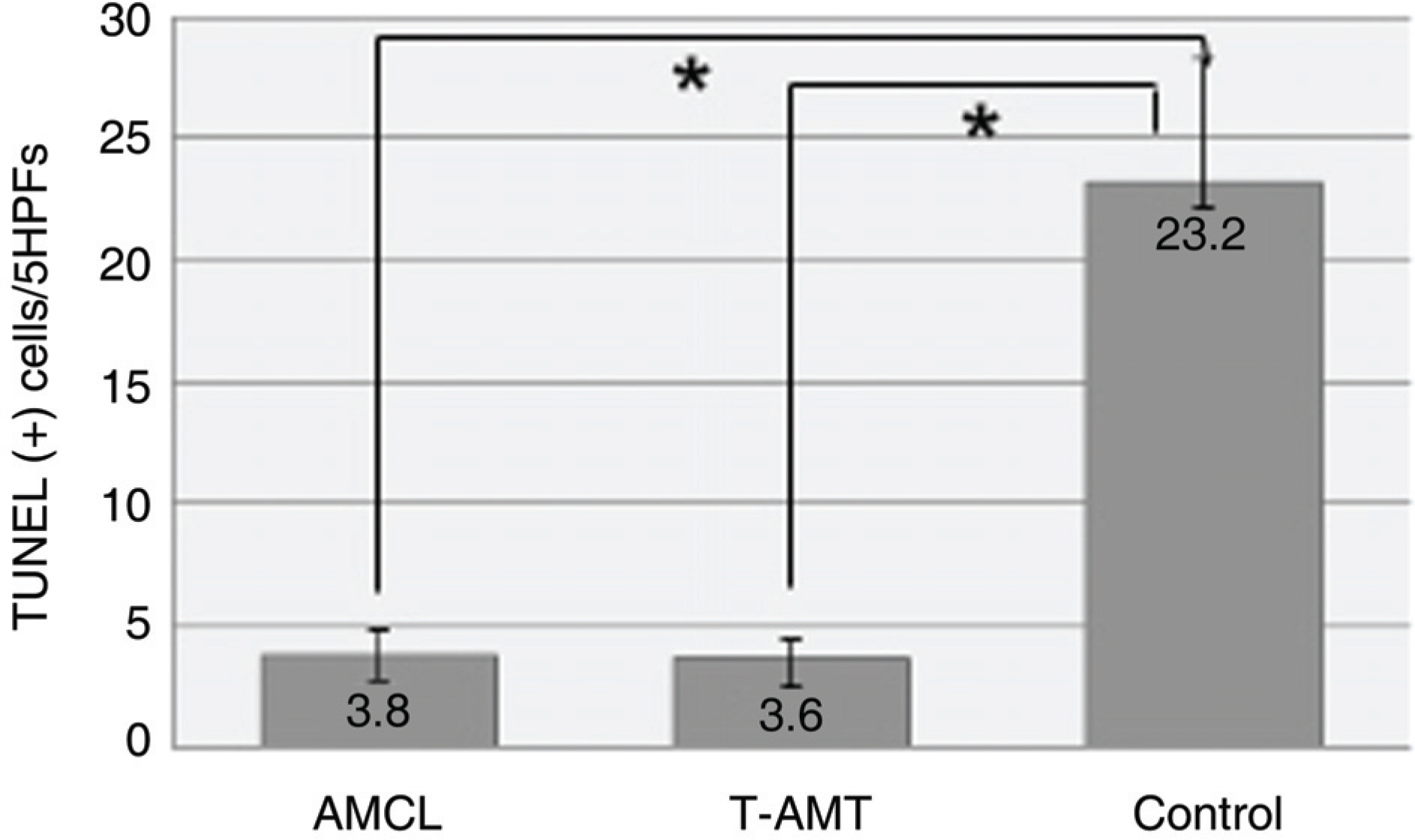
To investigate the efficacy of an amniotic membrane contact lens on corneal epithelial wound healing. METHODS: We made a model with a corneal epithelial wound by applying 6 mm round filter paper soaked with 1 N NaOHonto the central cornea in 24 eyes of 12 rabbits. The rabbits were divided into three groups: AMCL (amniotic membrane contact lens), T-AMT (temporary amniotic membrane transplantation) and the control group. We evaluated corneal wound healing every postoperative day using a digital photo slitlamp and fluorescein dye. The corneas were harvested for histopathologic studies after seven days and analyzed with hematoxylin-eosin (H & E) stain and TUNEL staining. RESULTS: The average wound healing time was similar between the amniotic membrane contact lens and the temporary amniotic membrane transplantation group. The number of the infiltrated PMNs (polymorphonuclear cells) was 8.8+/-2.58, 8.6+/-2.19 and 48.6+/-7.12 in the AMCL, T-AMT and control groups, respectively. Apoptotic keratocytes were 3.8+/-1.1, 3.6+/-1.09 and 23.2+/-5.06 in the AMCL, T-AMT and control groups, respectively. In the AMCL and T-AMT groups, the number of infiltrated PMNs and apoptotic keratocytes were significantly less than those the control group (p<0.05). There were not significant differences in the number of PMNs and apoptotic cells in the AMCL and the T-AMT groups. CONCLUSIONS: Amniotic membrane contact lenses have the benefits of being an easily applied method and having a wound healing ability comparable to that possible with conventional suture methods.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Wound Healing Effects of AmniSite-Lens in Rabbits
Tae Hyung Kim, Yeoun Sook Chun, Jae Chan Kim
J Korean Ophthalmol Soc. 2013;54(2):338-345. doi: 10.3341/jkos.2013.54.2.338.
Reference
-
References
1. Choi YS, Kim J-Y, Wee WR. Effect of the application of human amniotic membrane rabbit corneal wound healing after excimer laser photorefractive keratectomy. Cornea. 1998; 17:389–95.2. Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997; 123:303–12.
Article3. Chun DH, Jeon SL, Lee J-Y, Choi TH. The effect of amniotic membrane transplantation on corneal epithelial cell proliferation. J Korean Ophthalmol Soc. 2002; 43:1746–57.4. Taylor RJ, Wang MX. Rate of reepithelialization following amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 1998; 39:S1038.5. Fournier JH, McLachlan DL. Ocular surface reconstruction using amniotic membrane allograft for severe surface disorders in chemical burns: case report and review of the literature. Int Surg. 2005; 90:45–7.6. Jain S, Rastogi A. Evaluation of the outcome of amniotic membrane transplantation for ocular surface reconstruction in symblepharon. Eye. 2004; 18:1251–7.
Article7. John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am. 2003; 16:43–65.8. Barabino S, Rolando M, Bentivoglio G, et al. Role of amniotic membrane transplantation for conjunctival reconstruction in ocular-cicatricial pemphigoid. Ophthalmology. 2003; 110:474–80.
Article9. Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002; 109:1285–90.10. Tseng SC, Prabhasawat P, Lee SH. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997; 124:765–74.
Article11. Meller D, Tseng SC. In vitro conjunctival epithelial differentiation on preserved human amniotic membrane. Invest Ophthalmol Vis Sci. 1998; 39:S428.12. Tseng SC, Prabhasawat P, Barton K. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998; 116:431–41.
Article13. Taylor RJ, Wang MX. Rate of reepithelialization following amniotic membrane transplantation. Invest Ophthalmol Vis Sci. 1998; 39:S1038.14. Kim JS, Kim JC, Na BK, et al. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res. 2000; 70:329–37.
Article15. Buultmann S, You L, Spandau U, et al. Amniotic membrane down-regulate chemokine expression in human keratocytes. Invest Ophthalmol Vis Sci. 1999; 40:S3044.16. Tseng SC, Li DQ, Ma X. Downregulation of TGF-b1, b2, b3, and TGF-b receptor II expression in human corneal fibroblasts by amniotic membrane. Invest Ophthalmol Vis Sci. 1998; 39:S428.17. Rennekampff H-O, Dohrmann P, Fory R, Fandrich F. Evaluation of amniotic membrane as a adhesion prophylaxis in a novel surgical gastroschisis model. J Invest Surg. 1994; 7:187–93.18. Chin JR, Murphy G, Werb Z. Stromelsin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. J Biol Chem. 1985; 264:1367–76.19. Dogru M, Tsubota K. Current concepts in ocular surface reconstruction. Semin Ophthalmol. 2005; 20:75–93.
Article20. SIjiri S, Kobayashi A, Sugiyama K, Tseng SC. Evaluation of visual acuity and color vision in normal human eyes with a sutureless temporary amniotic membrane patch. Am J Ophthalmol. 2007; 144:938–42.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Wound Healing Effects of AmniSite-Lens in Rabbits
- Effect of Amniotic Membrane on Epithelial Thickness and Formation of Hemidesmosomes after Corneal Stromal Wound
- The Effect of Amniotic Membrane on Epithelial Wound Healing in Rabbit Cornea after Phototherapeutic Keratectomy
- Clinical Applications and Complications of Contact Lens Type Amniotic Membrane
- Human Amniotic Membrane Transplantation for Treatment of Fungal Ulcer