J Korean Surg Soc.
2011 Jun;80(6):404-411. 10.4174/jkss.2011.80.6.404.
Expression of the survivin-2B splice variant related to the progression of colorectal carcinoma
- Affiliations
-
- 1Department of Surgery, Soonchunhyang University College of Medicine, Cheonan, Korea. ssurge@sch.ac.kr
- 2Department of Pathology, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 3Department of Surgery, Dankook University Medical College, Cheonan, Korea.
- KMID: 2212185
- DOI: http://doi.org/10.4174/jkss.2011.80.6.404
Abstract
- PURPOSE
Recently, two alternatively spliced survivin variants, survivin-DeltaEx3 and survivin-2B, were identified in a single copy of the survivin gene. It has been reported that the expressions of survivin splice variants significantly correlates with the clinical results in many types of human carcinoma. We investigated the transcription levels of survivin and its splice variants in human colorectal carcinomas, and analyzed correlations between survivin expression levels and clinicopathologic features.
METHODS
We used Western blot and real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) to analyze the protein and mRNA expression levels of survivin variants in 51 colorectal carcinomas. The quantitative RT-PCR was performed using primer pairs specific for survivin and each of its splice variants, then normalized for the gene that encodes glyceraldehydes-3-phosphate dehydrogenase.
RESULTS
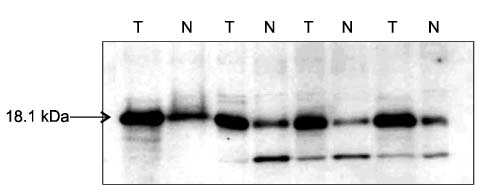
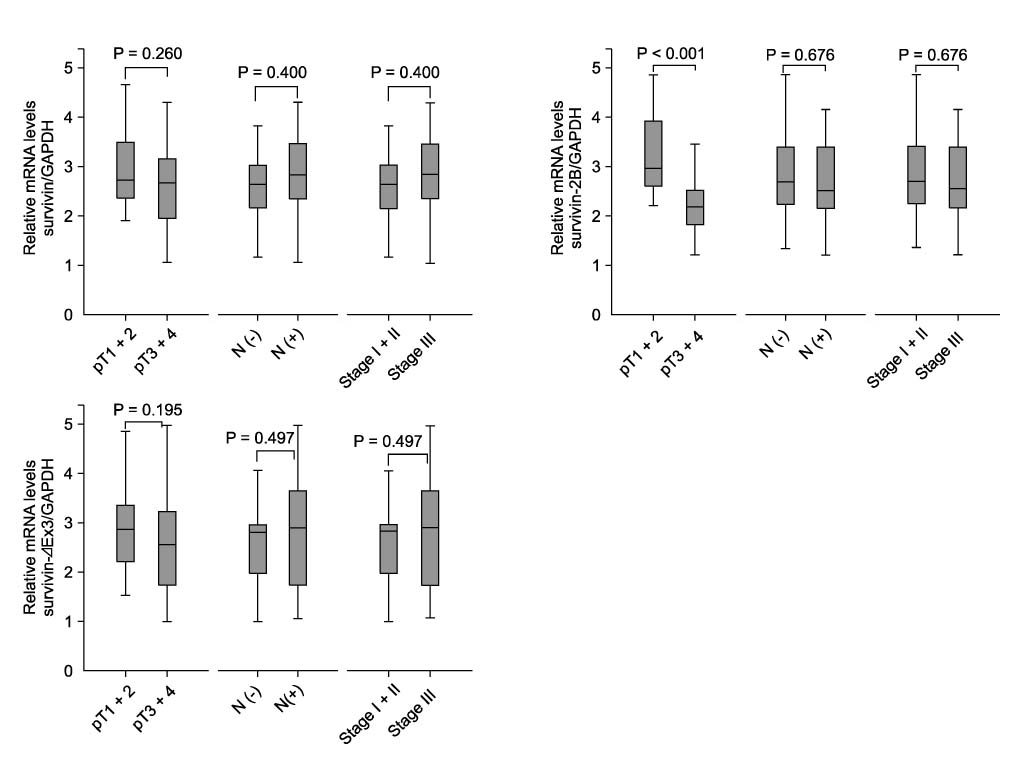
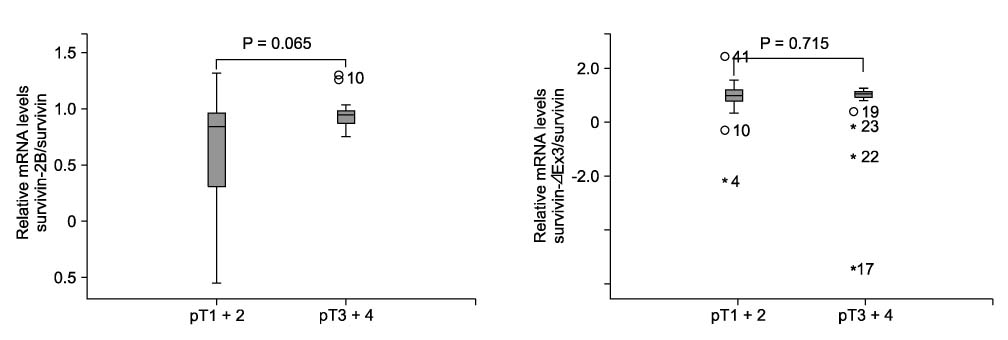
In Western blotting, the protein levels of survivin were higher in the tumor tissue than in normal tissue. The expression of survivin, survivin-2B and survivin-DeltaEx3 mRNA was present in 96%, 64.7%, and 82.4% of the samples, respectively. When the pathologic parameters were compared, colorectal cancers of advanced pT stages showed significant decrease in survivin-2B mRNA expression by the quantitative RT-PCR (P < 0.001).
CONCLUSION
The decreased expression of survivin-2B might be related to tumor progression in colorectal cancers. This finding indicates that alternatively spliced variants of survivin may be involved in refining the functions of survivin during tumor progression.
MeSH Terms
Figure
Reference
-
1. Modrek B, Lee C. A genomic view of alternative splicing. Nat Genet. 2002. 30:13–19.2. Ng CC, Koyama K, Okamura S, Kondoh H, Takei Y, Nakamura Y. Isolation and characterization of a novel TP53-inducible gene, TP53TG3. Genes Chromosomes Cancer. 1999. 26:329–335.3. Weng MW, Lai JC, Hsu CP, Yu KY, Chen CY, Lin TS, et al. Alternative splicing of MDM2 mRNA in lung carcinomas and lung cell lines. Environ Mol Mutagen. 2005. 46:1–11.4. Yukitake H, Furusawa M, Taira T, Iguchi-Ariga SM, Ariga H. AAT-1, a novel testis-specific AMY-1-binding protein, forms a quaternary complex with AMY-1, A-kinase anchor protein 84, and a regulatory subunit of cAMP-dependent protein kinase and is phosphorylated by its kinase. J Biol Chem. 2002. 277:45480–45492.5. Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997. 3:917–921.6. Li F. Role of survivin and its splice variants in tumorigenesis. Br J Cancer. 2005. 92:212–216.7. Islam A, Kageyama H, Hashizume K, Kaneko Y, Nakagawara A. Role of survivin, whose gene is mapped to 17q25, in human neuroblastoma and identification of a novel dominant-negative isoform, survivin-beta/2B. Med Pediatr Oncol. 2000. 35:550–553.8. Mahotka C, Krieg T, Krieg A, Wenzel M, Suschek CV, Heydthausen M, et al. Distinct in vivo expression patterns of survivin splice variants in renal cell carcinomas. Int J Cancer. 2002. 100:30–36.9. Meng H, Lu C, Mabuchi H, Tanigawa N. Prognostic significance and different properties of survivin splicing variants in gastric cancer. Cancer Lett. 2004. 216:147–155.10. Yamada Y, Kuroiwa T, Nakagawa T, Kajimoto Y, Dohi T, Azuma H, et al. Transcriptional expression of survivin and its splice variants in brain tumors in humans. J Neurosurg. 2003. 99:738–745.11. Nakagawa Y, Yamaguchi S, Hasegawa M, Nemoto T, Inoue M, Suzuki K, et al. Differential expression of survivin in bone marrow cells from patients with acute lymphocytic leukemia and chronic lymphocytic leukemia. Leuk Res. 2004. 28:487–494.12. O'Driscoll L, Linehan R, M Kennedy S, Cronin D, Purcell R, Glynn S, et al. Lack of prognostic significance of survivin, survivin-deltaEx3, survivin-2B, galectin-3, bag-1, bax-alpha and MRP-1 mRNAs in breast cancer. Cancer Lett. 2003. 201:225–236.13. Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res. 1998. 58:5071–5074.14. Chen WC, Liu Q, Fu JX, Kang SY. Expression of survivin and its significance in colorectal cancer. World J Gastroenterol. 2004. 10:2886–2889.15. Suga K, Yamamoto T, Yamada Y, Miyatake S, Nakagawa T, Tanigawa N. Correlation between transcriptional expression of survivin isoforms and clinicopathological findings in human colorectal carcinomas. Oncol Rep. 2005. 13:891–897.16. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995. 267:1456–1462.17. Rudin CM, Thompson CB. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997. 48:267–281.18. Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996. 88:386–401.19. Jäättelä M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999. 248:30–43.20. LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998. 17:3247–3259.21. Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997. 388:300–304.22. Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998. 58:5315–5320.23. Hague A, Moorghen M, Hicks D, Chapman M, Paraskeva C. BCL-2 expression in human colorectal adenomas and carcinomas. Oncogene. 1994. 9:3367–3370.24. Mahotka C, Wenzel M, Springer E, Gabbert HE, Gerharz CD. Survivin-deltaEx3 and survivin-2B: two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties. Cancer Res. 1999. 59:6097–6102.25. Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, et al. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001. 61:8664–8667.26. Krieg A, Mahotka C, Krieg T, Grabsch H, Müller W, Takeno S, et al. Expression of different survivin variants in gastric carcinomas: first clues to a role of survivin-2B in tumour progression. Br J Cancer. 2002. 86:737–743.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Change in Expression of Survivin Caused by Using Oxaliplatin in HCT116 Colon Cancer Cells
- Expression of Survivin and HSP90 in Colorectal Cancer and its Relationship with Clinicopathologic Factors
- Detection of Survivin and COX-2 in Thyroid Carcinoma: Anaplastic Carcinoma Shows Overexpression of Nuclear Survivin and Low COX-2 Expression
- The Expression of Cyclooxygenase-2 and Survivin in Urinary Bladder Transitional Cell Carcinoma
- Expression of Survivin and Its Correlation with Prognosis in Colorectal Cancer