J Periodontal Implant Sci.
2010 Jun;40(3):105-110. 10.5051/jpis.2010.40.3.105.
Biological effects of a semiconductor diode laser on human periodontal ligament fibroblasts
- Affiliations
-
- 1Department of Periodontology and Dental Research Institute, Seoul National University College of Dentistry, Seoul, Korea. periopf@snu.ac.kr
- KMID: 2212136
- DOI: http://doi.org/10.5051/jpis.2010.40.3.105
Abstract
- PURPOSE
It has been reported that low-level semiconductor diode lasers could enhance the wound healing process. The periodontal ligament is crucial for maintaining the tooth and surrounding tissues in periodontal wound healing. While low-level semiconductor diode lasers have been used in low-level laser therapy, there have been few reports on their effects on periodontal ligament fibroblasts (PDLFs). We performed this study to investigate the biological effects of semiconductor diode lasers on human PDLFs.
METHODS
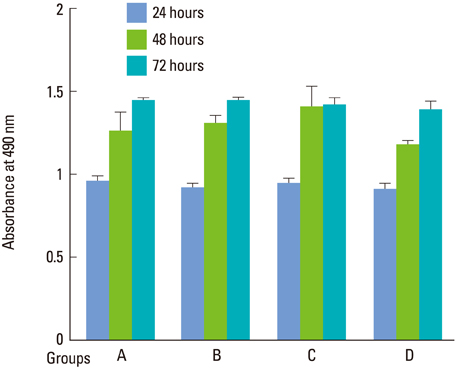
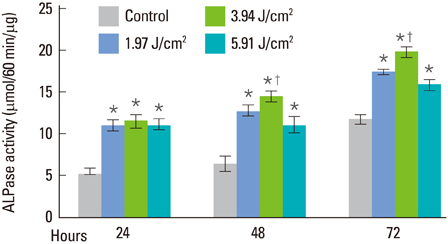
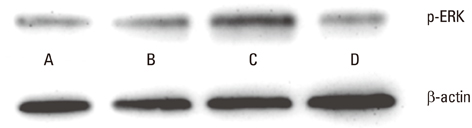
Human PDLFs were cultured and irradiated with a gallium-aluminum-arsenate (GaAlAs) semiconductor diode laser of which the wavelength was 810 nm. The power output was fixed at 500 mW in the continuous wave mode with various energy fluencies, which were 1.97, 3.94, and 5.91 J/cm2. A culture of PDLFs without laser irradiation was regarded as a control. Then, cells were additionally incubated in 72 hours for MTS assay and an alkaline phosphatase (ALPase) activity test. At 48 hours post-laser irradiation, western blot analysis was performed to determine extracellular signal-regulated kinase (ERK) activity. ANOVA was used to assess the significance level of the differences among groups (P<0.05).
RESULTS
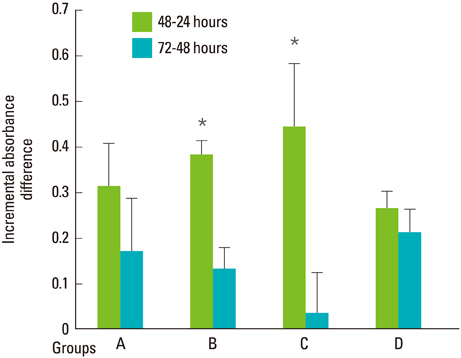
At all energy fluencies of laser irradiation, PDLFs proliferation gradually increased for 72 hours without any significant differences compared with the control over the entire period taken together. However, an increment of cell proliferation significantly greater than in the control occurred between 24 and 48 hours at laser irradiation settings of 1.97 and 3.94 J/cm2 (P<0.05). The highest ALPase activity was found at 48 and 72 hours post-laser irradiation with 3.94 J/cm2 energy fluency (P<0.05). The phosphorylated ERK level was more prominent at 3.94 J/cm2 energy fluency than in the control.
CONCLUSIONS
The present study demonstrated that the GaAlAs semiconductor diode laser promoted proliferation and differentiation of human PDLFs.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Blotting, Western
Cell Proliferation
Extracellular Signal-Regulated MAP Kinases
Fibroblasts
Humans
Low-Level Light Therapy
Lasers, Semiconductor
Periodontal Ligament
Phosphotransferases
Semiconductors
Tooth
Wound Healing
Alkaline Phosphatase
Extracellular Signal-Regulated MAP Kinases
Phosphotransferases
Figure
Reference
-
1. Midda M. Lasers in periodontics. Newsl Int Acad Periodontol. 1991; 1:2–3.2. Midda M. Lasers in periodontics. Periodontal Clin Investig. 1992; 14:14–20.3. Midda M. The use of lasers in periodontology. Curr Opin Dent. 1992; 2:104–108.4. Ando Y, Aoki A, Watanabe H, Ishikawa I. Bactericidal effect of erbium YAG laser on periodontopathic bacteria. Lasers Surg Med. 1996; 19:190–200.
Article5. Aoki A, Sasaki KM, Watanabe H, Ishikawa I. Lasers in non-surgical periodontal therapy. Periodontol 2000. 2004; 36:59–97.
Article6. Folwaczny M, Mehl A, Aggstaller H, Hickel R. Antimicrobial effects of 2.94 microm Er:YAG laser radiation on root surfaces: an in vitro study. J Clin Periodontol. 2002; 29:73–78.
Article7. Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, et al. Treatment of periodontal pockets with a diode laser. Lasers Surg Med. 1998; 22:302–311.
Article8. Kreisler M, Al Haj H, d'Hoedt B. Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med. 2005; 37:350–355.
Article9. Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation: a review. J Clin Periodontol. 1996; 23:492–496.
Article10. Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971; 122:532–535.
Article11. Reddy GK, Stehno-Bittel L, Enwemeka CS. Laser photostimulation accelerates wound healing in diabetic rats. Wound Repair Regen. 2001; 9:248–255.
Article12. Pinfildi CE, Liebano RE, Hochman BS, Ferreira LM. Helium-neon laser in viability of random skin flap in rats. Lasers Surg Med. 2005; 37:74–77.
Article13. Herascu N, Velciu B, Calin M, Savastru D, Talianu C. Low-level laser therapy (LLLT) efficacy in post-operative wounds. Photomed Laser Surg. 2005; 23:70–73.
Article14. Isaka J, Ohazama A, Kobayashi M, Nagashima C, Takiguchi T, Kawasaki H, et al. Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol. 2001; 72:314–323.
Article15. Lekic P, McCulloch CA. Periodontal ligament cell population: the central role of fibroblasts in creating a unique tissue. Anat Rec. 1996; 245:327–341.
Article16. Kreisler M, Christoffers AB, Willershausen B, d'Hoedt B. Effect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: an in vitro study. J Clin Periodontol. 2003; 30:353–358.
Article17. Kreisler M, Meyer C, Stender E, Daublander M, Willershausen-Zonnchen B, d'Hoedt B. Effect of diode laser irradiation on the attachment rate of periodontal ligament cells: an in vitro study. J Periodontol. 2001; 72:1312–1317.
Article18. do Nascimento PM, Pinheiro AL, Salgado MA, Ramalho LM. A preliminary report on the effect of laser therapy on the healing of cutaneous surgical wounds as a consequence of an inversely proportional relationship between wave-length and intensity: histological study in rats. Photomed Laser Surg. 2004; 22:513–518.
Article19. Lopes-Martins RA, Albertini R, Martins PS, Bjordal JM, Faria Neto HC. Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by carrageenan. Photomed Laser Surg. 2005; 23:377–381.
Article20. Correa F, Lopes Martins RA, Correa JC, Iversen VV, Joenson J, Bjordal JM. Low-level laser therapy (GaAs lambda = 904 nm) reduces inflammatory cell migration in mice with lipopolysaccharide-induced peritonitis. Photomed Laser Surg. 2007; 25:245–249.
Article21. Kreisler M, Christoffers AB, Al-Haj H, Willershausen B, d'Hoedt B. Low level 809-nm diode laser-induced in vitro stimulation of the proliferation of human gingival fibroblasts. Lasers Surg Med. 2002; 30:365–369.
Article22. Pereira AN, Eduardo Cde P, Matson E, Marques MM. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med. 2002; 31:263–267.
Article23. Stein A, Benayahu D, Maltz L, Oron U. Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg. 2005; 23:161–166.
Article24. Shimizu N, Yamaguchi M, Goseki T, Shibata Y, Takiguchi H, Iwasawa T, et al. Inhibition of prostaglandin E2 and interleukin 1-beta production by low-power laser irradiation in stretched human periodontal ligament cells. J Dent Res. 1995; 74:1382–1388.
Article25. Giannopoulou C, Cimasoni G. Functional characteristics of gingival and periodontal ligament fibroblasts. J Dent Res. 1996; 75:895–902.
Article26. Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996; 84:345–357.
Article27. Kim TI, Jang JH, Lee YM, Rhyu IC, Chung CP, Han SB, et al. Biomimetic approach on human periodontal ligament cells using synthetic oligopeptides. J Periodontol. 2004; 75:925–932.
Article28. Ozawa Y, Shimizu N, Kariya G, Abiko Y. Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone. 1998; 22:347–354.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The comparison clinical study effect of Diode Laser & Ms coat usage for dentinal hypersensitivity following Periodontal therapy
- Comparison of Photocoagulation with the Argon and Diode Laser in Rabbit Eyes
- Biological Characteristics of Human Periodontal Ligament Cells
- Effect of Inorganic Polyphosphate on Cultured Periodontal Ligament Cells
- A study on immunocytochemical localization of fibronectin during cytodifferentiation of periodontal ligament fibroblasts in the beagle dogs