J Korean Surg Soc.
2010 Jun;78(6):357-368. 10.4174/jkss.2010.78.6.357.
Recipient's Killer Cell Immunoglobulin-like Receptor Genotype and Human Leukocyte Antigen C Ligand Influence the Clinical Outcome following Living Donor Liver Transplantation
- Affiliations
-
- 1Department of Surgery, Daejeon St. Mary's Hospital, The Catholic University of Korea, School of Medicine, Daejeon, Korea.
- 2Department of Surgery, Seoul St. Mary's Hospital, The Catholic University of Korea, School of Medicine, Seoul, Korea. kimdg@catholic.ac.kr
- 3Department of Microbiology, Seoul St. Mary's Hospital, The Catholic University of Korea, School of Medicine, Seoul, Korea.
- 4Hematopoietic Stem Cell Bank, Seoul St. Mary's Hospital, The Catholic University of Korea, School of Medicine, Seoul, Korea.
- 5Department of Pathology, Seoul St. Mary's Hospital, The Catholic University of Korea, School of Medicine, Seoul, Korea.
- KMID: 2211966
- DOI: http://doi.org/10.4174/jkss.2010.78.6.357
Abstract
- PURPOSE
The design of this study was to determine the most influential factor(s) on post-transplant immunological consequences, particularly with regard to the role of killer cell immunoglobulin-like receptors (KIRs) and their ligands (type I human leukocyte antigen (HLA)) in unstable liver function.
METHODS
Retrospectively collected data from 319 recipients undergoing adult living donor liver transplantation (LDLT) using a right lobe graft between January 2002 and August 2008 were analyzed. Patients were categorized according to the serum alanine transaminase (ALT) pattern; stable ALT pattern was defined as ALT pattern during 3 months post-transplantation, except for initial 2 weeks post-transplantation, in which 2 times or less additional elevation(s) of serum alanine transaminase (ALT) (> or =80 IU/L) were observed. When a serum ALT pattern showed fluctuating and/or unpredictable nature, it was defined as an unstable pattern. In addition, genetic information of KIRs and HLA-C allotypes received from 68 recipients and 59 donors was analyzed by way of polymerase chain reaction using sequence-specific primers (PCR-SSP) to determine the factor(s) influencing a serum ALT pattern.
RESULTS
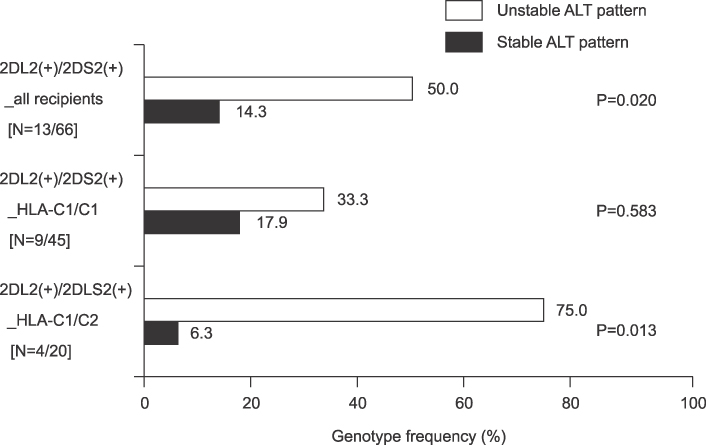
Among 319 LDLT recipients included in this study, the actual incidences of AR and unstable ALT pattern were 13.4% (43/319) and 42.3% (135/319), respectively. Unstable ALT pattern correlated with poorer survival following LDLT than stable pattern (P<0.000). Genetically, unstable ALT pattern was related to recipients carrying KIR2DL2(+)/KIR2DS2(+) combined with the heterogeneous HLA-C allotype (HLA-C1/C2), (relative risks 45.0, 95% confidence interval 2.160~937.321; P=0.013).
CONCLUSION
This study indicates that, when performing LDLT, pretransplant determination of recipient's KIRs and HLA-C allotypes may be beneficial in coping with post-transplant immunological circumstances.
Keyword
MeSH Terms
Figure
Reference
-
1. Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997. 63:243–249.2. Sandborn WJ, Hay JE, Porayko MK, Gores GJ, Steers JL, Krom RA, et al. Cyclosporine withdrawal for nephrotoxicity in liver transplant recipients does not result in sustained improvement in kidney function and causes cellular and ductopenic rejection. Hepatology. 1994. 19:925–932.3. Golling M, Frankenberg MV, Hofmann WJ, Lohse A, Herfarth C, Otto G. Cyclosporine A reduction and withdrawal in liver transplantation: a risk-benefit analysis. Transplant Proc. 1997. 29:2819–2821.4. Devlin J, Doherty D, Thomson L, Wong T, Donaldson P, Portmann B, et al. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology. 1998. 27:926–933.5. Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001. 72:449–454.6. Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002. 20:217–251.7. Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005. 5:201–214.8. Moya-Quiles MR, Muro M, Torio A, Sanchez-Bueno F, Miras M, Marin L, et al. Human leukocyte antigen-C in short- and long-term liver graft acceptance. Liver Transpl. 2003. 9:218–227.9. Moretta A, Biassoni R, Bottino C, Pende D, Vitale M, Poggi A, et al. Major histocompatibility complex class I-specific receptors on human natural killer and T lymphocytes. Immunol Rev. 1997. 155:105–117.10. Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat Rev Immunol. 2004. 4:190–198.11. Boyington JC, Brooks AG, Sun PD. Structure of killer cell immunoglobulin-like receptors and their recognition of the class I MHC molecules. Immunol Rev. 2001. 181:66–78.12. Biassoni R, Falco M, Cambiaggi A, Costa P, Verdiani S, Pende D, et al. Amino acid substitutions can influence the natural killer (NK)-mediated recognition of HLA-C molecules. Role of serine-77 and lysine-80 in the target cell protection from lysis mediated by "group 2" or "group 1" NK clones. J Exp Med. 1995. 182:605–609.13. Demetris AJ, Seaberg EC, Batts KP, Ferrell LD, Ludwig J, Markin RS, et al. Reliability and predictive value of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database nomenclature and grading system for cellular rejection of liver allografts. Hepatology. 1995. 21:408–416.14. Ormonde DG, de Boer WB, Kierath A, Bell R, Shilkin KB, House AK, et al. Banff schema for grading liver allograft rejection: utility in clinical practice. Liver Transpl Surg. 1999. 5:261–268.15. Bunce M, Welsh KI. Rapid DNA typing for HLA-C using sequence-specific primers (PCR-SSP): identification of serological and non-serologically defined HLA-C alleles including several new alleles. Tissue Antigens. 1994. 43:7–17.16. Gomez-Lozano N, Vilches C. Genotyping of human killer-cell immunoglobulin-like receptor genes by polymerase chain reaction with sequence-specific primers: an update. Tissue Antigens. 2002. 59:184–193.17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. 240:205–213.18. Hiby SE, Walker JJ, O'Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004. 200:957–965.19. Ruggeri L, Capanni M, Mancusi A, Martelli MF, Velardi A. The impact of donor natural killer cell alloreactivity on allogeneic hematopoietic transplantation. Transpl Immunol. 2005. 14:203–206.20. Suzuki Y, Hamamoto Y, Ogasawara Y, Ishikawa K, Yoshikawa Y, Sasazuki T, et al. Genetic polymorphisms of killer cell immunoglobulin-like receptors are associated with susceptibility to psoriasis vulgaris. J Invest Dermatol. 2004. 122:1133–1136.21. van der Slik AR, Koeleman BP, Verduijn W, Bruining GJ, Roep BO, Giphart MJ. KIR in type 1 diabetes: disparate distribution of activating and inhibitory natural killer cell receptors in patients versus HLA-matched control subjects. Diabetes. 2003. 52:2639–2642.22. Cook MA, Milligan DW, Fegan CD, Darbyshire PJ, Mahendra P, Craddock CF, et al. The impact of donor KIR and patient HLA-C genotypes on outcome following HLA-identical sibling hematopoietic stem cell transplantation for myeloid leukemia. Blood. 2004. 103:1521–1526.23. Momot T, Koch S, Hunzelmann N, Krieg T, Ulbricht K, Schmidt RE, et al. Association of killer cell immunoglobulin-like receptors with scleroderma. Arthritis Rheum. 2004. 50:1561–1565.24. Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999. 163:2314–2321.25. Liu ZX, Govindarajan S, Okamoto S, Dennert G. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J Immunol. 2000. 164:6480–6486.26. Moya-Quiles MR, Alvarez R, Miras M, Gomez-Mateo J, Lopez-Alvarez MR, Marin-Moreno I, et al. Impact of recipient HLA-C in liver transplant: a protective effect of HLA-Cw*07 on acute rejection. Hum Immunol. 2007. 68:51–58.27. Bishara A, Brautbar C, Zamir G, Eid A, Safadi R. Impact of HLA-C and Bw epitopes disparity on liver transplantation outcome. Hum Immunol. 2005. 66:1099–1105.28. Hanvesakul R, Spencer N, Cook M, Gunson B, Hathaway M, Brown R, et al. Donor HLA-C genotype has a profound impact on the clinical outcome following liver transplantation. Am J Transplant. 2008. 8:1931–1941.29. Tran TH, Middleton D, Dohler B, Scherer S, Meenagh A, Sleator C, et al. Reassessing the impact of donor HLA-C genotype on long-term liver transplant survival. Am J Transplant. 2009. 9:1674–1678.30. Mendel JB, Chavin KD, Bratton C, Knechtle SJ. HLA-C and liver transplant outcomes: interpreting the facts. Am J Transplant. 2009. 9:1491–1492.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Leukocyte Antigen-C Genotype and Killer Immunoglobulin-like Receptor-Ligand Matching in Korean Living Donor Liver Transplantation
- A First Experience of Rh(D) Incompatible Living Related Liver Transplantation in Korea
- Liver stiffness measurement and outcomes of living donor liver transplantation
- Maternal killer-cell immunoglobulin-like receptors and paternal human leukocyte antigen ligands in recurrent pregnancy loss cases in Turkey
- Complete transition from open to laparoscopic living donor hepatectomy: 8-year experience with more than 500 laparoscopy cases