J Pathol Transl Med.
2016 Mar;50(2):129-137. 10.4132/jptm.2015.12.24.
Interobserver Variability of Ki-67 Measurement in Breast Cancer
- Affiliations
-
- 1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. sypmd@snu.ac.kr
- 2Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2211380
- DOI: http://doi.org/10.4132/jptm.2015.12.24
Abstract
- BACKGROUND
As measurement of Ki-67 proliferation index is an important part of breast cancer diagnostics, we conducted a multicenter study to examine the degree of concordance in Ki-67 counting and to find factors that lead to its variability.
METHODS
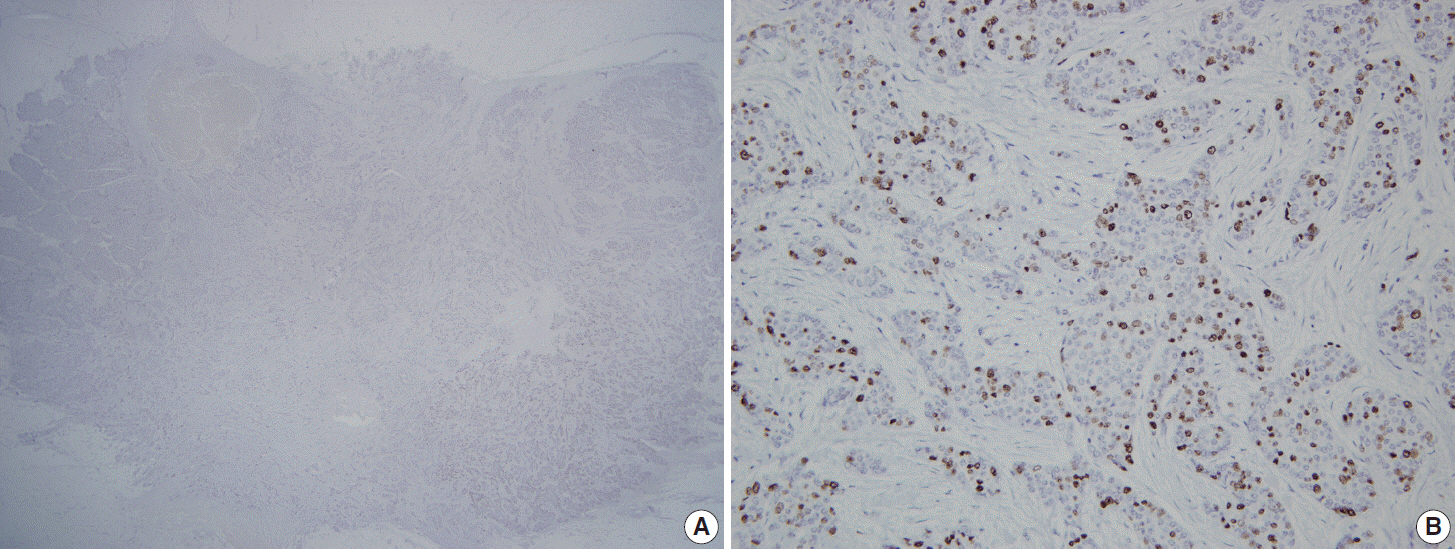
Thirty observers from thirty different institutions reviewed Ki-67-stained slides of 20 different breast cancers on whole sections and tissue microarray (TMA) by online system. Ten of the 20 breast cancers had hot spots of Ki-67 expression. Each observer scored Ki-67 in two different ways: direct counting (average vs. hot spot method) and categorical estimation. Intraclass correlation coefficient (ICC) of Ki-67 index was calculated for comparative analysis.
RESULTS
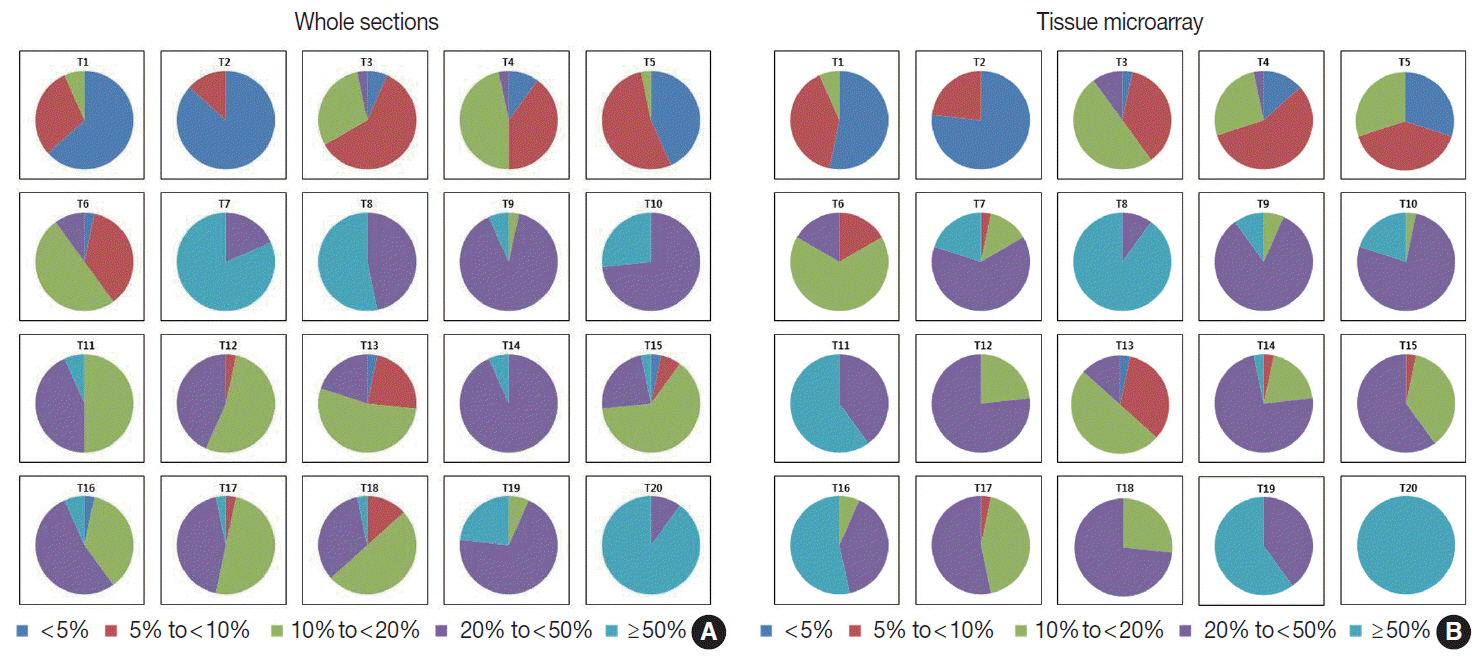
For direct counting, ICC of TMA was slightly higher than that of whole sections using average method (0.895 vs 0.858). The ICC of tumors with hot spots was lower than that of tumors without (0.736 vs 0.874). In tumors with hot spots, observers took an additional counting from the hot spot; the ICC of whole sections using hot spot method was still lower than that of TMA (0.737 vs 0.895). In categorical estimation, Ki-67 index showed a wide distribution in some cases. Nevertheless, in tumors with hot spots, the range of distribution in Ki-67 categories was decreased with hot spot method and in TMA platform.
CONCLUSIONS
Interobserver variability of Ki-67 index for direct counting and categorical estimation was relatively high. Tumors with hot spots showed greater interobserver variability as opposed to those without, and restricting the measurement area yielded lower interobserver variability.
Keyword
Figure
Reference
-
1. Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007; 117:3155–63.
Article2. SØrlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–74.3. Perou CM, SØrlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–52.
Article4. Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004; 10:5367–74.
Article5. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006; 295:2492–502.
Article6. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–50.
Article7. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013; 24:2206–23.8. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.9. Bruno S, Darzynkiewicz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992; 25:31–40.
Article10. Luporsi E, André F, Spyratos F, et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012; 132:895–915.
Article11. Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010; 11:174–83.
Article12. Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in post-menopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008; 26:5569–75.
Article13. Penault-Llorca F, André F, Sagan C, et al. Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009; 27:2809–15.
Article14. Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007; 99:167–70.
Article15. Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011; 103:1656–64.16. Polley MY, Leung SC, McShane LM, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013; 105:1897–906.17. Wong SC, Chan JK, Lo ES, et al. The contribution of bifunctional SkipDewax pretreatment solution, rabbit monoclonal antibodies, and polymer detection systems in immunohistochemistry. Arch Pathol Lab Med. 2007; 131:1047–55.
Article18. Zabaglo L, Salter J, Anderson H, et al. Comparative validation of the SP6 antibody to Ki67 in breast cancer. J Clin Pathol. 2010; 63:800–4.
Article19. Ekholm M, Beglerbegovic S, Grabau D, et al. Immunohistochemical assessment of Ki67 with antibodies SP6 and MIB1 in primary breast cancer: a comparison of prognostic value and reproducibility. Histopathology. 2014; 65:252–60.
Article20. Fasanella S, Leonardi E, Cantaloni C, et al. Proliferative activity in human breast cancer: Ki-67 automated evaluation and the influence of different Ki-67 equivalent antibodies. Diagn Pathol. 2011; 6 Suppl 1:S7.
Article21. Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005; 16:1723–39.
Article22. Mohammed ZM, McMillan DC, Elsberger B, et al. Comparison of visual and automated assessment of Ki-67 proliferative activity and their impact on outcome in primary operable invasive ductal breast cancer. Br J Cancer. 2012; 106:383–8.
Article23. Laurinavicius A, Plancoulaine B, Laurinaviciene A, et al. A methodology to ensure and improve accuracy of Ki67 labelling index estimation by automated digital image analysis in breast cancer tissue. Breast Cancer Res. 2014; 16:R35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Value of the S-phase Fraction and Ki-67 Expression in Early Breast Cancer
- Ki-67 as a Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
- Cobb Angle Measurement of Scoliosis Using Computer Measurement of Digitally Acquired Radiographs: Intraobserver and Interobserver Variability
- CT-based quantitative evaluation of radiation-induced lung fibrosis: a study of interobserver and intraobserver variations
- Proliferative Markers of Breast Cancer