J Nutr Health.
2015 Oct;48(5):390-397. 10.4163/jnh.2015.48.5.390.
Effects of chronic alcohol and excessive iron intake on mitochondrial DNA damage in the rat liver
- Affiliations
-
- 1Department of Food and Nutrition, Kyung Hee University, Seoul 02447, Korea. jchung@khu.ac.kr
- KMID: 2209873
- DOI: http://doi.org/10.4163/jnh.2015.48.5.390
Abstract
- PURPOSE
In this study, we investigated the effects of chronic alcohol and excessive iron intake on mitochondrial DNA (mtDNA) damage and the progression of alcoholic liver injury in rats.
METHODS
Twenty-four Sprague-Dawley male rats were divided into four groups (Control, EtOH, Fe, and EtOH + Fe), and fed either control or ethanol (36% of total calories) liquid diet with or without 0.6% carbonyl iron for eight weeks. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities, liver malondialdehyde concentrations were measured by colorimetric assays. Liver histopathology was examined by Hematoxylin-eosin staining of the fixed liver tissues. The integrity of the hepatic mtDNA and nuclear DNA was measured by long-range PCR. The gene expression levels of cytochrome c oxidase subunit 1 (Cox1) and NADH dehydrogenase subunit 4 (Nd4) were examined by real-time PCR.
RESULTS
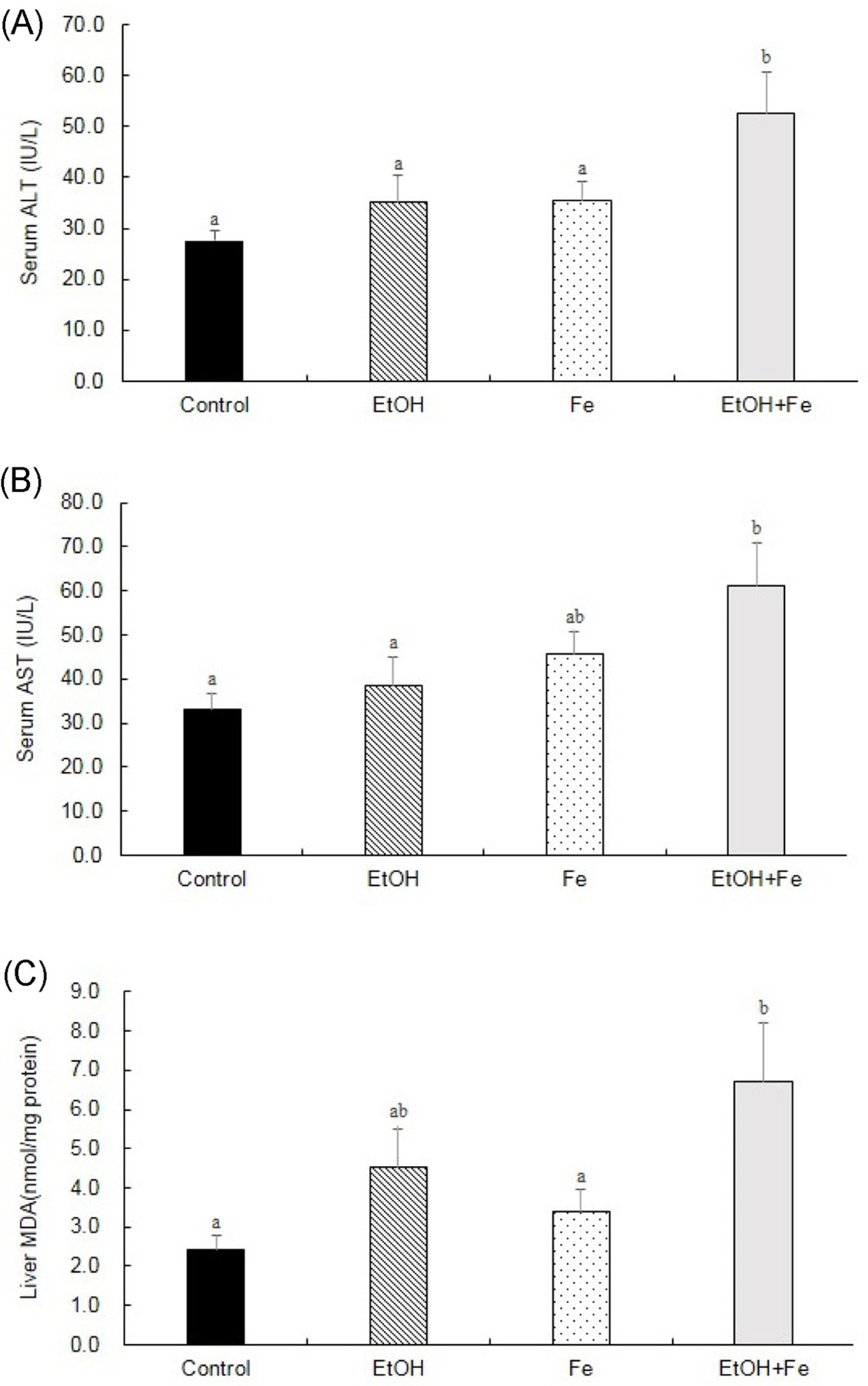
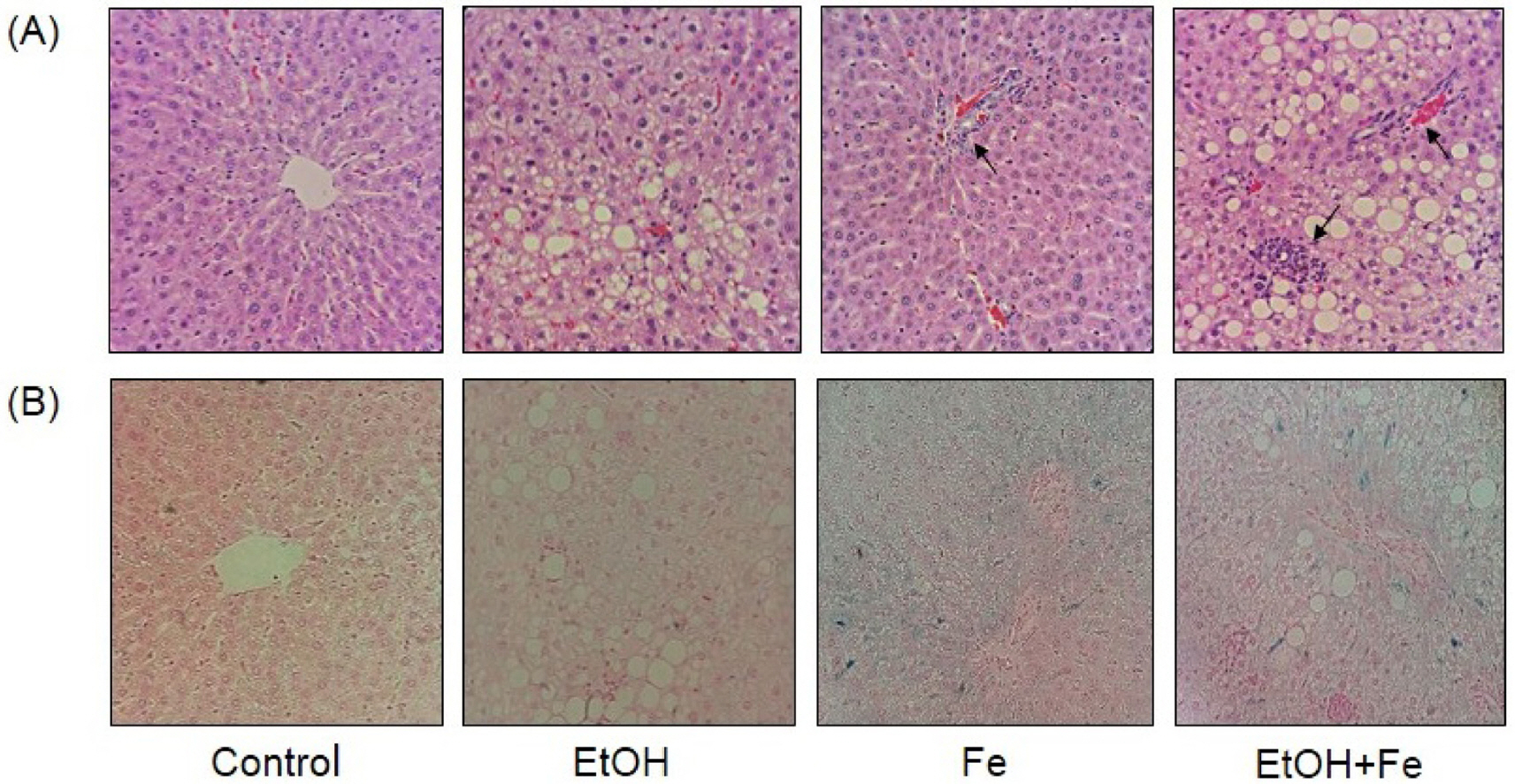
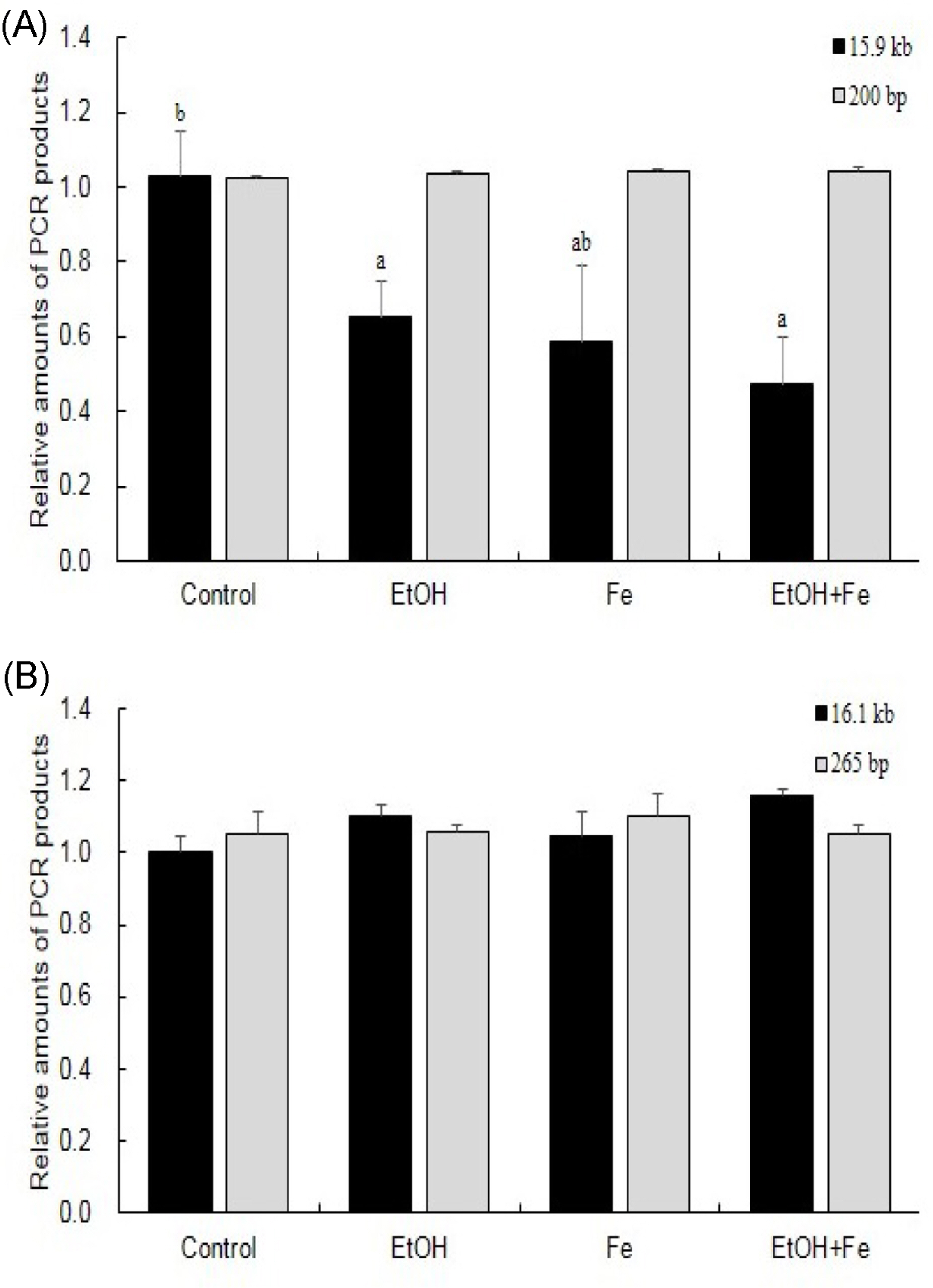
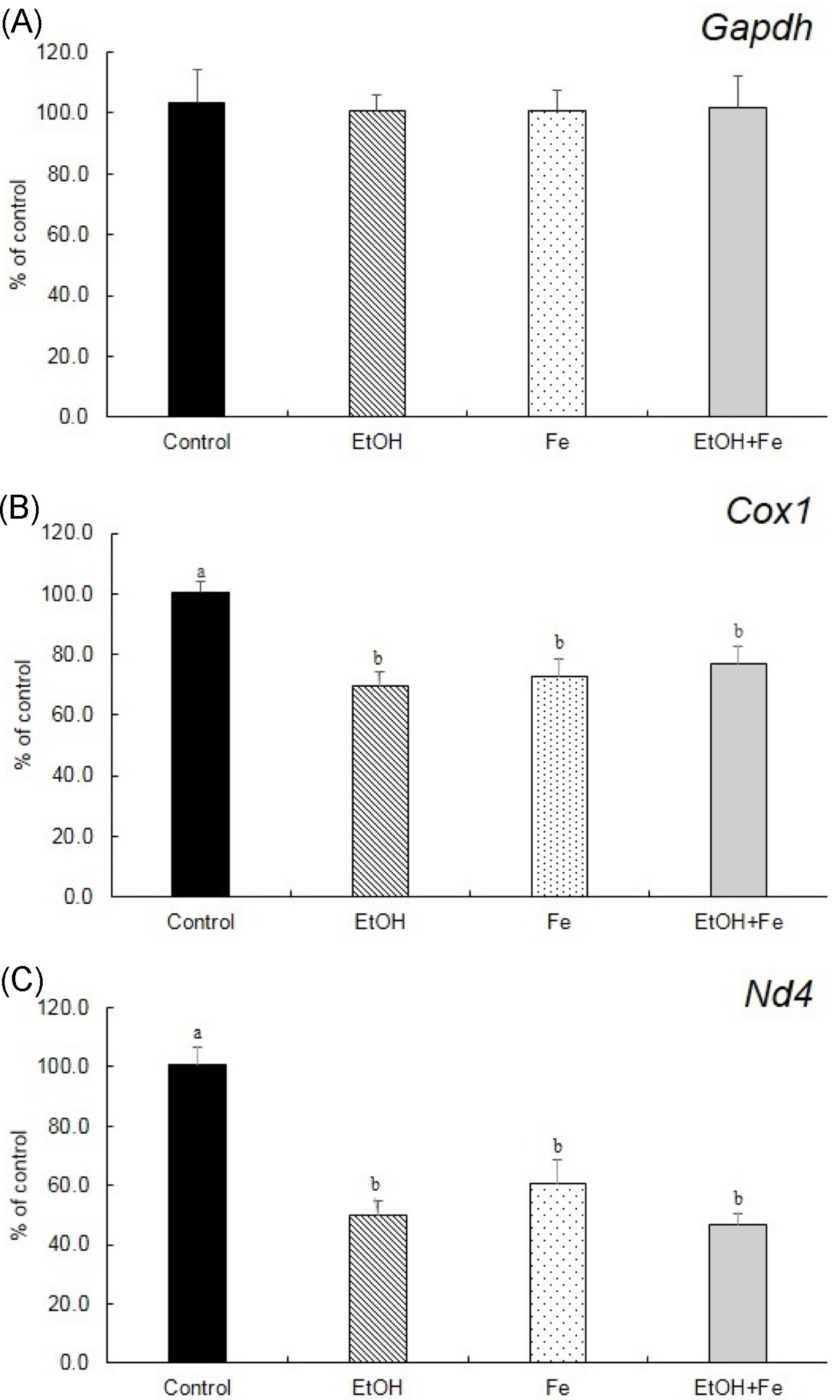
Serum ALT and AST activities were significantly higher in the EtOH+Fe group, as compared to the Control group. Similarly, among four groups, liver histology showed the most severe lipid accumulation, inflammation, and necrosis in the EtOH + Fe group. PCR amplification of near-full-length (15.9 kb) mtDNA showed more than 50% loss of full-length product in the liver of the EtOH + Fe group, whereas amounts of PCR products of a nuclear DNA were unaffected. In addition, the changes in the mtDNA integrity showed correlation with reductions in the mRNA levels of mitochondrial gene Cox1 and Nd4.
CONCLUSION
Our data suggested that the liver injury associated with excessive iron and alcohol intake involved mtDNA damage and corresponding mitochondrial dysfunction.
Keyword
MeSH Terms
-
Alanine Transaminase
Alcoholics
Animals
Aspartate Aminotransferases
Diet
DNA
DNA, Mitochondrial*
Electron Transport Complex IV
Ethanol
Gene Expression
Genes, Mitochondrial
Humans
Inflammation
Iron*
Liver*
Male
Malondialdehyde
NADH Dehydrogenase
Necrosis
Polymerase Chain Reaction
Rats*
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
RNA, Messenger
Alanine Transaminase
Aspartate Aminotransferases
DNA
DNA, Mitochondrial
Electron Transport Complex IV
Ethanol
Iron
Malondialdehyde
NADH Dehydrogenase
RNA, Messenger
Figure
Reference
-
1.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2013: Korea National Health and Nutrition Examination Survey (KNHANES VI-1). Cheongju: Korea Centers for Disease Control and Prevention;2014.2.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Community health survey 2014. Cheongju; Korea Centers for Disease Control and Prevention:. 2015.3.Statistice Korea. Cause of death statistics 2013 [Internet]. Daejeon: Statistice Korea;2014. [cited 2015 Aug 1]. Available form:. http://kostat.go.kr.4.Stewart S., Jones D., Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001. 7(9):408–413.
Article5.Becker U., Deis A., Sørensen TI., Grønbaek M., Borch-Johnsen K., Müller CF., Schnohr P., Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996. 23(5):1025–1029.
Article6.Valerio LG Jr., Parks T., Petersen DR. Alcohol mediates increases in hepatic and serum nonheme iron stores in a rat model for alcohol-induced liver injury. Alcohol Clin Exp Res. 1996. 20(8):1352–1361.
Article7.Tsukamoto H., Horne W., Kamimura S., Niemelä O., Parkkila S., Ylä-Herttuala S., Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995. 96(1):620–630.
Article8.Ganne-Carrié N., Christidis C., Chastang C., Ziol M., Chapel F., Imbert-Bismut F., Trinchet JC., Guettier C., Beaugrand M. Liver iron is predictive of death in alcoholic cirrhosis: a multivariate study of 229 consecutive patients with alcoholic and/or hepatitis C virus cirrhosis: a prospective follow up study. Gut. 2000. 46(2):277–282.9.Kim HT., Chun SS., Joung SH., Yun ME. Nutrient intake status of Korean drinkers: analysis of data from Korea National Health and Nutrition Examination Survey (KNHANES), 2011. J Korean Diet Assoc. 2013. 19(4):343–355.
Article10.Kwak CS., Lee JW., Hyun WJ. The effects of smoking and alcohol drinking on nutritional status and eating habits in adult males. Korean J Community Nutr. 2000. 5(2):161–171.11.Whitfield JB., Zhu G., Heath AC., Powell LW., Martin NG. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res. 2001. 25(7):1037–1045.
Article12.Blake R., Trounce IA. Mitochondrial dysfunction and complications associated with diabetes. Biochim Biophys Acta. 2014. 1840(4):1404–1412.
Article13.Aliev G., Priyadarshini M., Reddy VP., Grieg NH., Kaminsky Y., Cacabelos R., Ashraf GM., Jabir NR., Kamal MA., Nikolenko VN., Zamyatnin AA Jr., Benberin VV., Bachurin SO. Oxidative stress mediated mitochondrial and vascular lesions as markers in the pathogenesis of Alzheimer disease. Curr Med Chem. 2014. 21(19):2208–2217.
Article14.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012. 12(10):685–698.
Article15.Boland ML., Chourasia AH., Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013. 3:292.
Article16.Olynyk J., Hall P., Reed W., Williams P., Kerr R., Mackinnon M. A long-term study of the interaction between iron and alcohol in an animal model of iron overload. J Hepatol. 1995. 22(6):671–676.
Article17.Takeyama Y., Kamimura S., Kuroiwa A., Sohda T., Irie M., Shijo H., Okumura M. Role of Kupffer cell-derived reactive oxygen intermediates in alcoholic liver disease in rats in vivo. Alcohol Clin Exp Res. 1996. 20(9 Suppl):335A–339A.
Article18.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95(2):351–358.
Article19.Ayala-Torres S., Chen Y., Svoboda T., Rosenblatt J., Van Houten B. Analysis of gene-specific DNA damage and repair using quantitative polymerase chain reaction. Methods. 2000. 22(2):135–147.
Article20.Yakes FM., Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997. 94(2):514–519.
Article21.Masola B., Devlin TM. Intramitochondrial localization of alanine aminotransferase in rat-liver mitochondria: comparison with glutaminase and aspartate aminotransferase. Amino Acids. 1995. 9(4):363–374.
Article22.Demori I., Voci A., Fugassa E., Burlando B. Combined effects of high-fat diet and ethanol induce oxidative stress in rat liver. Alcohol. 2006. 40(3):185–191.
Article23.Brandon-Warner E., Schrum LW., Schmidt CM., McKillop IH. Rodent models of alcoholic liver disease: of mice and men. Alcohol. 2012. 46(8):715–725.
Article24.Mathews S., Xu M., Wang H., Bertola A., Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014. 306(10):G819–G823.
Article25.Day CP., James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998. 114(4):842–845.
Article26.Wu D., Cederbaum AI. Oxidative stress mediated toxicity exerted by ethanol-inducible CYP2E1. Toxicol Appl Pharmacol. 2005. 207(2 Suppl):70–76.
Article27.Xiong S., She H., Sung CK., Tsukamoto H. Iron-dependent activation of NF-κB in Kupffer cells: a priming mechanism for alcoholic liver disease. Alcohol. 2003. 30(2):107–113.
Article28.Choi JS., Koh IU., Lee HJ., Kim WH., Song J. Effects of excess dietary iron and fat on glucose and lipid metabolism. J Nutr Bio-chem. 2013. 24(9):1634–1644.
Article29.Silva M., da Costa Guerra JF., Sampaio AF., de Lima WG., Silva ME., Pedrosa ML. Iron dextran increases hepatic oxidative stress and alters expression of genes related to lipid metabolism contributing to hyperlipidaemia in murine model. Biomed Res Int. 2015. 2015:272617.
Article30.Richter C., Park JW., Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988. 85(17):6465–6467.
Article31.Cederbaum A. Nrf2 and antioxidant defense against CYP2E1 toxicity. Expert Opin Drug Metab Toxicol. 2009. 5(10):1223–1244.
Article32.Loguercio C., Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003. 34(1):1–10.
Article33.Lu Y., Cederbaum AI. CYP2E1 potentiation of LPS and TNFα-induced hepatotoxicity by mechanisms involving enhanced oxidative and nitrosative stress, activation of MAP kinases, and mitochondrial dysfunction. Genes Nutr. 2010. 5(2):149–167.
Article34.Larosche I., Choumar A., Fromenty B., Lettéron P., Abbey-Toby A., Van Remmen H., Epstein CJ., Richardson A., Feldmann G., Pessayre D., Mansouri A. Prolonged ethanol administration depletes mitochondrial DNA in MnSOD-overexpressing transgenic mice, but not in their wild type littermates. Toxicol Appl Pharmacol. 2009. 234(3):326–338.
Article35.Tang Y., Gao C., Xing M., Li Y., Zhu L., Wang D., Yang X., Liu L., Yao P. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol. 2012. 50(5):1194–1200.
Article36.Lakshmi Devi S., Anuradha CV. Mitochondrial damage, cytotoxicity and apoptosis in iron-potentiated alcoholic liver fibrosis: amelioration by taurine. Amino Acids. 2010. 38(3):869–879.
Article37.Gao X., Campian JL., Qian M., Sun XF., Eaton JW. Mitochondrial DNA damage in iron overload. J Biol Chem. 2009. 284(8):4767–4775.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alcohol and hepatocarcinogenesis
- Effect of Chronic Alcohol Intake on Vasopressin and Oxytocin-containing Neurons in the Paraventricular and Supraoptic Nucleus of the Rat Hypothalamus
- Iron Supplementation Reverses the Reduction of Hydroxymethylcytosine in Hepatic DNA Associated With Chronic Alcohol Consumption in Rats
- A Survey on Nutrient Intake of University Students by Alcohol Intake
- The effect of moderate alcohol drinking in nonalcoholic fatty liver disease