J Korean Soc Transplant.
2015 Sep;29(3):139-147. 10.4285/jkstn.2015.29.3.139.
Culture with Growth Factor Supplements Improves the Viability and Function of Rat Hepatocytes
- Affiliations
-
- 1Department of Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. kimdg@catholic.ac.kr
- 2Department of Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2202586
- DOI: http://doi.org/10.4285/jkstn.2015.29.3.139
Abstract
- BACKGROUND
To identify the optimum culture conditions by investigating isolated rat hepatocytes cultured in medium containing different growth factors.
METHODS
Hepatocytes were isolated from rats using a two-step perfusion technique and divided into the following four groups cultured in medium containing different growth factors: control, epidermal growth factor (EGF), insulin, and EGF+insulin. The viability of the cultured rat hepatocytes and liver function parameters, including albumin, ammonia, and urea in the culture medium, were measured. Hepatocyte morphology was examined by staining with hematoxylin and eosin, and albumin receptor expression was confirmed by immunofluorescence.
RESULTS
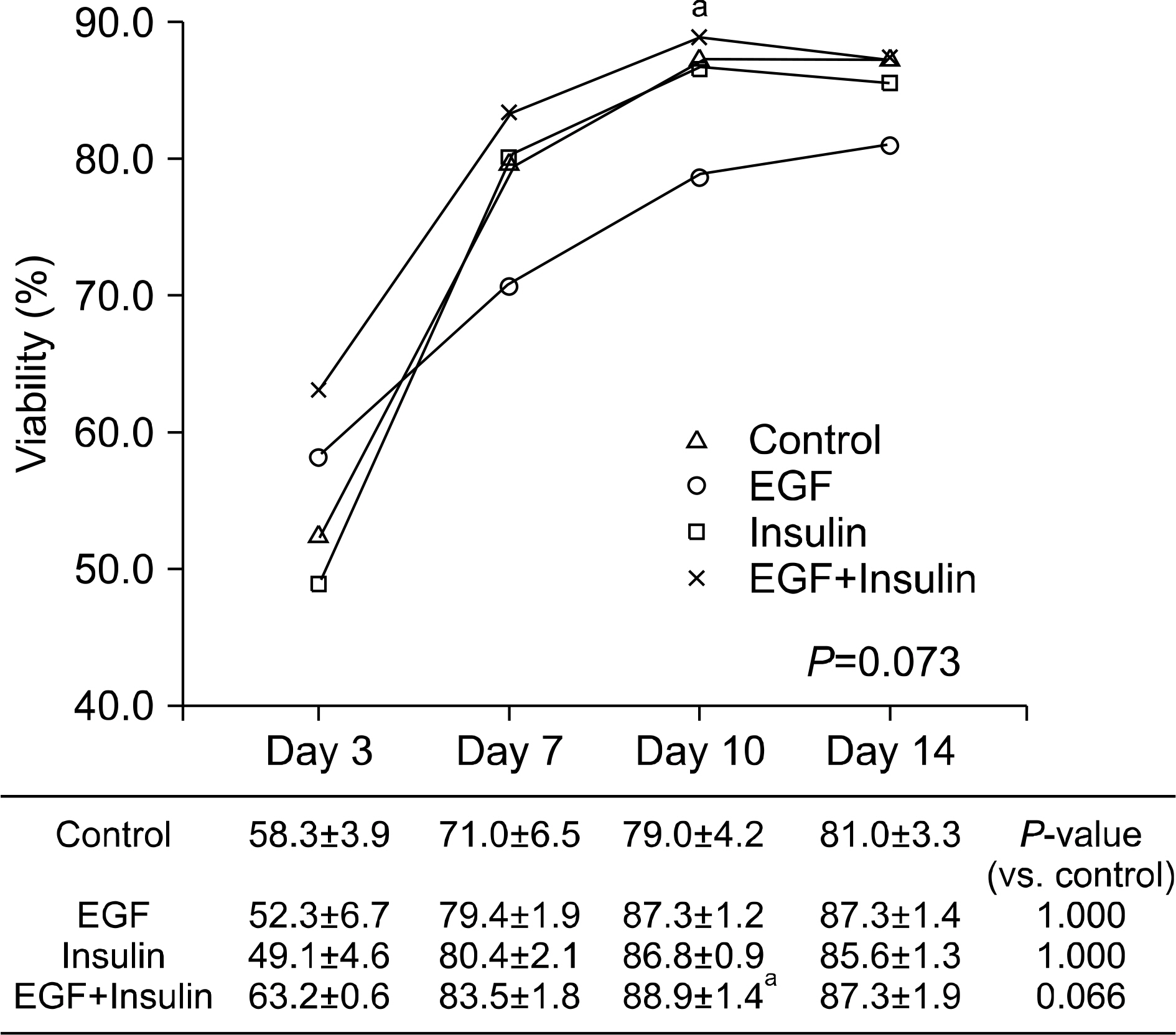
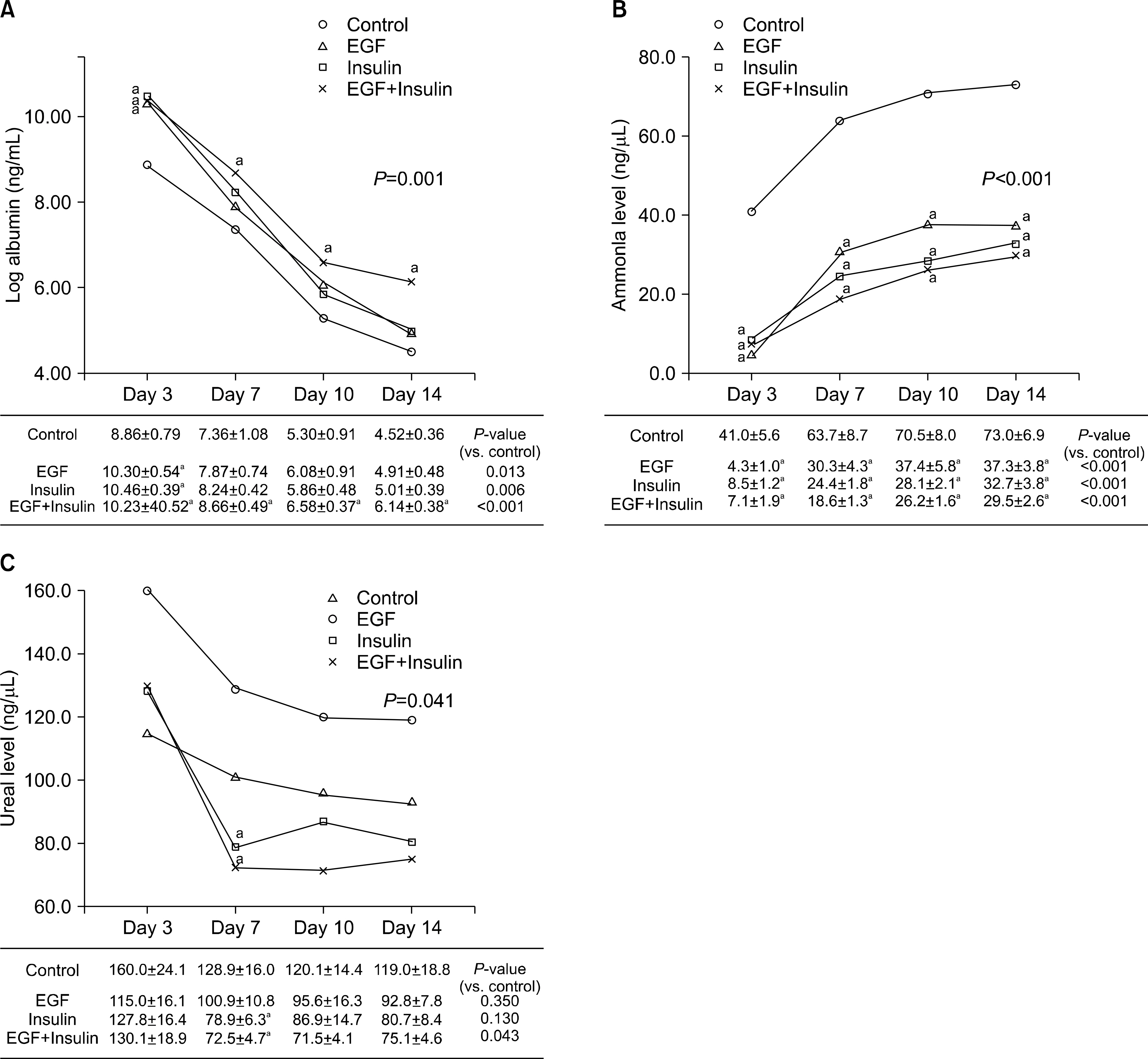
Slightly higher viability was observed in the growth factor groups than in the control group, although without significance (P=0.073). The levels of albumin (P=0.001), ammonia (P<0.001), and urea (P=0.041) differed significantly among the four groups. The functional parameters in the growth factor groups, particularly the EGF+insulin group, were significantly superior to those in the control group. The morphology of the hepatocytes in all growth factor groups was well maintained at 10 days. However, the control group showed deterioration in cell morphology by day 7.
CONCLUSIONS
Morphological and functional assessment indicated that the presence of growth factors, particularly EGF+insulin, provided culture conditions superior to those of non-supplemented medium.
Keyword
MeSH Terms
-
Ammonia
Animals
Eosine Yellowish-(YS)
Epidermal Growth Factor
Fluorescent Antibody Technique
Hematoxylin
Hepatocytes*
Insulin
Intercellular Signaling Peptides and Proteins
Liver
Perfusion
Rats*
Receptors, Albumin
Urea
Ammonia
Eosine Yellowish-(YS)
Epidermal Growth Factor
Hematoxylin
Insulin
Intercellular Signaling Peptides and Proteins
Receptors, Albumin
Urea
Figure
Reference
-
References
1). O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993; 342:273–5.2). Gill RQ, Sterling RK. Acute liver failure. J Clin Gastroenterol. 2001; 33:191–8.
Article3). O'Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014; 60:663–70.4). Neuberger J, James O. Guidelines for selection of patients for liver transplantation in the era of donor-organ shortage. Lancet. 1999; 354:1636–9.
Article5). Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013; 369:2525–34.
Article6). Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012; 93:342–7.
Article7). Bumgardner GL, Fasola C, Sutherland DE. Prospects for hepatocyte transplantation. Hepatology. 1988; 8:1158–61.
Article8). Fox IJ, Chowdhury JR. Hepatocyte transplantation. Am J Transplant. 2004; 4(Suppl 6):7–13.
Article9). Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997; 63:559–69.
Article10). Strom SC, Bruzzone P, Cai H, Ellis E, Lehmann T, Mitamura K, et al. Hepatocyte transplantation: clinical experience and potential for future use. Cell Transplant. 2006; 15(Suppl 1):S105–10.
Article11). Mitaka T. The current status of primary hepatocyte culture. Int J Exp Pathol. 1998; 79:393–409.
Article12). Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976; 13:29–83.13). Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006; 82:441–9.
Article14). Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, et al. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976; 192:892–4.15). Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992; 24:3052–3.
Article16). Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010; 7:288–98.
Article17). Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science. 2000; 287:1258–62.
Article18). Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000; 6:1229–34.
Article19). Demetriou AA, Brown RS Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004; 239:660–7.
Article20). Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969; 43:506–20.21). Hughes RD, Mitry RR, Dhawan A. Hepatocyte transplantation for metabolic liver disease: UK experience. J R Soc Med. 2005; 98:341–5.
Article22). Richman RA, Claus TH, Pilkis SJ, Friedman DL. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1976; 73:3589–93.
Article23). McGowan JA, Strain AJ, Bucher NL. DNA synthesis in primary cultures of adult rat hepatocytes in a defined medium: effects of epidermal growth factor, insulin, glucagon, and cyclic-AMP. J Cell Physiol. 1981; 108:353–63.
Article24). Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997; 276:60–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rat-Hepatocyte Culture and Differentiation in Hormone-Supplemented Media
- Effects of glucose concentration in the medium on rat hepatocyte culture
- Cryopreservation of Primary Hepatocytes for Repeated Investigational Uses
- Cryopreservation of Rat Hepatocytes for the Use of Primary Culture
- Optimal Number of Hepatocytes per Microcarrier in Spheroid Culture using Cytodex 3 Microcarrier