J Korean Soc Transplant.
2012 Dec;26(4):269-276. 10.4285/jkstn.2012.26.4.269.
Liver Transplantation for Hepatitis C Virus-Related Liver Disease in Korea
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. kwleegs@gmail.com
- 2Department of Surgery, Seoul National University Boramae Hospital, Seoul, Korea.
- 3Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- 4Department of Surgery, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Surgery, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 6Department of Surgery, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Center for Liver Cancer, National Cancer Center, Goyang, Korea.
- 8Department of Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 9Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2202430
- DOI: http://doi.org/10.4285/jkstn.2012.26.4.269
Abstract
- BACKGROUND
A management protocol for hepatitis C virus (HCV) after liver transplantation (LT) has not been established in Korea. We therefore investigated HCV transplant protocols and post-transplant results from liver transplant centers in Korea.
METHODS
The HCV protocol and medical data of individual cases from eight major liver transplant centers were compiled and analyzed.
RESULTS
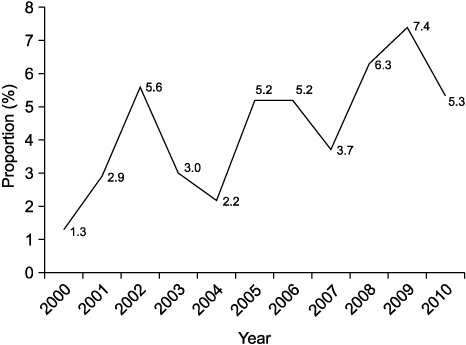
A post-transplant protocol biopsy was performed in only three centers. In these centers, HCV treatment was considered when pathological abnormalities were confirmed on the protocol biopsy (irrespective of liver function). In the other five centers, biopsies were performed when biochemical parameters were aggravated. Only two out of the eight centers performed preemptive or prophylactic therapy. A total of 5,663 adult LTs were performed between 2000 and 2010. HCV-related liver disease was responsible for 277 LTs (4.9%). Pre-transplant data were not available in many patients, including HCV genotype and serum HCV RNA level. Tacrolimus was more frequently used for initial maintenance immunosuppression than cyclosporine A (61.7% vs. 36.8%). Post-transplant HCV treatment was performed in 135 patients (48.7%). Sixty-seven recipients (24.2%) died during follow-up after LT and 11 HCV-related graft loss (4.0%) developed. The cumulative patient survival rate was 74.7% at 5 years and 67.9% at 10 years after LT.
CONCLUSIONS
The HCV management protocol after LT varied markedly between the eight Korean transplant centers and a standard protocol did not exist. A nationwide multicenter study is required to investigate the most effective treatment for HCV after LT, with the goal of establishing the most effective standard protocol.
MeSH Terms
Figure
Cited by 2 articles
-
The Management of HCV Recurrence after Liver Transplantation
YoungRok Choi, Kwang-Woong Lee
J Korean Soc Transplant. 2013;27(2):37-41. doi: 10.4285/jkstn.2013.27.2.37.Outcomes for Patients with Hepatitis C Virus after Liver Transplantation in Korea
Jong Man Kim
J Korean Soc Transplant. 2016;30(4):155-164. doi: 10.4285/jkstn.2016.30.4.155.
Reference
-
1. Guillouche P, Féray C. Systematic review: anti-viral therapy of recurrent hepatitis C after liver transplantation. Aliment Pharmacol Ther. 2011. 33:163–174.
Article2. Féray C, Samuel D, Thiers V, Gigou M, Pichon F, Bismuth A, et al. Reinfection of liver graft by hepatitis C virus after liver transplantation. J Clin Invest. 1992. 89:1361–1365.
Article3. Wright TL, Donegan E, Hsu HH, Ferrell L, Lake JR, Kim M, et al. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992. 103:317–322.
Article4. Prieto M, Berenguer M, Rayón JM, Córdoba J, Argüello L, Carrasco D, et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999. 29:250–256.
Article5. Berenguer M. Natural history of recurrent hepatitis C. Liver Transpl. 2002. 8:10 Suppl 1. S14–S18.
Article6. Gane EJ, Naoumov NV, Qian KP, Mondelli MU, Maertens G, Portmann BC, et al. A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology. 1996. 110:167–177.
Article7. Féray C, Gigou M, Samuel D, Paradis V, Wilber J, David MF, et al. The course of hepatitis C virus infection after liver transplantation. Hepatology. 1994. 20:1137–1143.
Article8. Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996. 334:815–820.
Article9. Berenguer M, Ferrell L, Watson J, Prieto M, Kim M, Rayón M, et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol. 2000. 32:673–684.
Article10. Picciotto FP, Tritto G, Lanza AG, Addario L, De Luca M, Di Costanzo GG, et al. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007. 46:459–465.
Article11. Charlton M, Seaberg E, Wiesner R, Everhart J, Zetterman R, Lake J, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology. 1998. 28:823–830.
Article12. Everson GT, Trotter J, Forman L, Kugelmas M, Halprin A, Fey B, et al. Treatment of advanced hepatitis C with a low accelerating dosage regimen of antiviral therapy. Hepatology. 2005. 42:255–262.
Article13. Watt K, Veldt B, Charlton M. A practical guide to the management of HCV infection following liver transplantation. Am J Transplant. 2009. 9:1707–1713.
Article14. Ishii N, Watashi K, Hishiki T, Goto K, Inoue D, Hijikata M, et al. Diverse effects of cyclosporine on hepatitis C virus strain replication. J Virol. 2006. 80:4510–4520.
Article15. Trotter JF. Hot-topic debate on hepatitis C virus: the type of immunosuppression matters. Liver Transpl. 2011. 17:Suppl 3. S20–S23.
Article16. Berenguer M, Royuela A, Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007. 13:21–29.
Article17. Wiesner RH. A long-term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998. 66:493–499.
Article18. McAlister VC, Haddad E, Renouf E, Malthaner RA, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006. 6:1578–1585.
Article19. Carrión JA, Navasa M, García-Retortillo M, García-Pagan JC, Crespo G, Bruguera M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007. 132:1746–1756.
Article20. Selzner N, Renner EL, Selzner M, Adeyi O, Kashfi A, Therapondos G, et al. Antiviral treatment of recurrent hepatitis C after liver transplantation: predictors of response and long-term outcome. Transplantation. 2009. 88:1214–1221.
Article21. Cescon M, Grazi GL, Cucchetti A, Vetrone G, Ravaioli M, Ercolani G, et al. Predictors of sustained virological response after antiviral treatment for hepatitis C recurrence following liver transplantation. Liver Transpl. 2009. 15:782–789.
Article22. ReViS-TC Study Group. Cyclosporine a-based immunosuppression reduces relapse rate after antiviral therapy in transplanted patients with hepatitis C virus infection: a large multicenter cohort study. Transplantation. 2011. 92:334–340.23. Chalasani N, Manzarbeitia C, Ferenci P, Vogel W, Fontana RJ, Voigt M, et al. Peginterferon alfa-2a for hepatitis C after liver transplantation: two randomized, controlled trials. Hepatology. 2005. 41:289–298.
Article24. Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002. 122:889–896.
Article25. Berenguer M, Prieto M, San Juan F, Rayón JM, Martinez F, Carrasco D, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002. 36:202–210.
Article26. Neumann UP, Berg T, Bahra M, Puhl G, Guckelberger O, Langrehr JM, et al. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation. 2004. 77:226–231.
Article27. Steinmüller T, Seehofer D, Rayes N, Müller AR, Settmacher U, Jonas S, et al. Increasing applicability of liver transplantation for patients with hepatitis B-related liver disease. Hepatology. 2002. 35:1528–1535.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The management of chronic hepatitis C
- Indication and survival among liver transplant patients in Yangon Speciality Hospital, Myanmar
- A case of fulminant hepatic failure complicating hepatitis A virus superinfection in a hepatitis B virus carrier
- Understanding Acute Liver Failure: A Basic Overview of Definition and Treatment
- The Role of Hepatitis Virus and Alcohol in Chronic Liver Disease