J Korean Soc Endocrinol.
2005 Oct;20(5):519-523. 10.3803/jkes.2005.20.5.519.
A case of Methimazole-Induced Cholestatic Jaundice With Agranulocytosis
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Presbyterian Medical Center, Cheonju, Korea.
- KMID: 2200601
- DOI: http://doi.org/10.3803/jkes.2005.20.5.519
Abstract
- Methimazole is a widely used and generally well-tolerated antithyroid agent. Adverse reactions occur in 1~5% of patients taking methimazole medication, but these are most commonly transient, benign leukopenia and a skin rash. Severe cholestatic jaundice, combined with agranulocytosis, has been known as a rare complication. Herein, a case of methimazole induced cholestatic jaundice, with agranulocytosis, is reported.
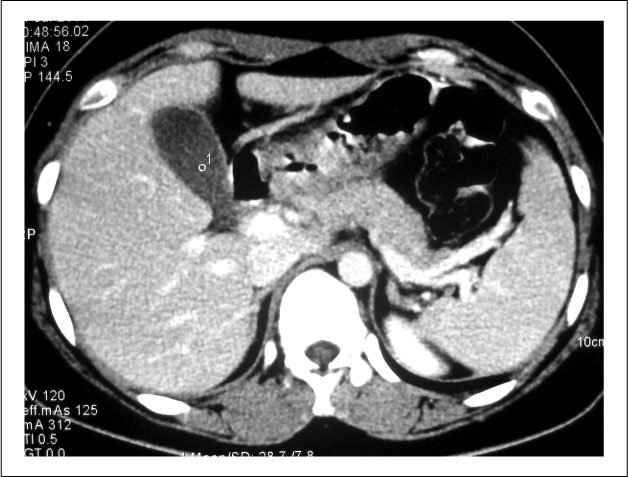
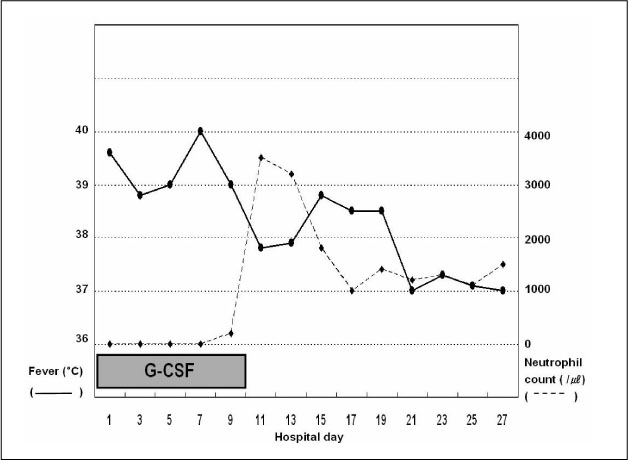
Figure
Reference
-
1. Marisa CW, Joao HR, Nazareth B, Rubens SW, Chady SF. Adverse Effects Related to Thionamide Drugs and their dose regimen. Am J Med Sci. 1989. 297:216–219.3. Cooper DS, Goldminz D, Levin AA. Agranulocytosis associated with antithyroid drugs. Effects of patient age and drug dose. Ann Inern Med. 1983. 98:26–29.5. Mikhail NE. Methimazole-induced cholestatic jaundice. South Med J. 2004. 97:178–182.6. Specht NW, Boehme EJ. Death due to agranulocytosis induced by methimazole therapy. JAMA. 1952. 149:1010–1011.7. Rosenbaum H, Reveno WS. Agranulocytosis and toxic hepatitis from methimazole. JAMA. 1953. 152:27.8. Shipp J. Jaundice during methimazole(Tapazole) administration. Ann Intern Med. 1955. 42:701–706.9. Manojlovic D, Nesovic M, Micic J, Duric D. Agranulocytose ot hepatite chronique chez les toalades traites par favistan. Srpskl Archiv Za Celokupno Lekarstro. 1977. 105:549–554.10. International agranulocytosis and aplastic anemia study group. Risk of agranulocytosis and aplastic anemia in relation to use of antithyroid drugs. Br Med J. 1988. 297:262–265.11. Douer D, Eisenstein Z. Methimazole-induced agranulocytosis: growth inhibition of myeloid progenitor cells by the patient's serum. Eur J Haematol. 1988. 40:91–94.12. Palmblad J, Johnson B, Kanerud L. Treatment of drug-induced agranulocytosis with recombinant granulocyte, macropahge-colony stimulating factor. J Intern Med. 1990. 228:537–542.13. Heinrich B, Gross M, Gobel FD. Methimazole- Induced Agranulocytosis and Granulocyte-Colony Stimulating Factor. Ann of Int Med. 1989. 111:621–622.14. Fukata S, Kuma K, Sugawara M. Granulocyte colony-stimulating factor(G-CSF) dose not improve recovery from antithyroid drug-induced agranulocytosis: a prospective study. Thyroid. 1999. 9:29–31.15. Vitug AC, Goldman JM. Hepatotoxicity from antithyroid drugs. Hrom Res. 1985. 21:229–234.16. Becker CE, Gorden P, Robbins J. Hepatitis from methimazole during adrenal steroid therapy for malignant exophthalmos. JAMA. 1968. 206:1787–1789.17. AntaonAranda E. Intrahepatic cholestasis in untreated hyperthyroidism. Rev Esp Enferm Dig. 2000. 92:49–50.18. Kravetz D. Intrahepatic cholestasis caused by danantizol. Rev Esp Enferm Apar Dig. 1972. 38:725–738.19. Fisher MG, Nayer HR, Miller A. Methimazole-induced jaundice. JAMA. 1973. 223:1028–1029.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Methimazole-Induced Cholestatic Jaundice with Steroid Therapy
- A Case of Antithyroid Drug-Induced Agranulocytosis Treated with Granulocyte Colony-Stimulating Factor (G-CSF) and Methylprednisolone
- A Case of Methimazole-induced Agranulocytosis and Kikuchi's Disease in a Patient with Graves' Disease
- A case of propylthiouracil-induced hepatitis with agranulocytosis
- A Case of Acute Appendicitis in a Patient with Methimazole-Induced Agranulocytosis