J Lipid Atheroscler.
2012 Jun;1(1):11-20. 10.12997/jla.2012.1.1.11.
The Peroxisome Proliferator-Activated Receptor delta Agonist, GW501516, Inhibits Angiogenesis through Dephosphorylation of Endothelial Nitric Oxide Synthase
- Affiliations
-
- 1Department of Pediatrics, Seoul Metropolitan Children's Hospital, Seoul, Korea.
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, College of Medicine, Chung-Ang University, Seoul, Korea. jtkim@cau.ac.kr
- KMID: 2198371
- DOI: http://doi.org/10.12997/jla.2012.1.1.11
Abstract
OBJECTIVE
Peroxisome proliferator-activated receptor delta (PPAR-delta) is an ubiquitously expressed nuclear receptor that has been implicated in adipose tissue formation, brain development, and atherosclerosis. Despite mouse studies demonstrating that PPAR-delta activation has favorable anti-atherogenic properties by improving systemic lipid profiles, the relationship between PPAR-delta agonist and angiogenesis is unknown. We hypothesized that PPAR-delta ligands modulate the angiogenesis.
METHODS
To test this hypothesis we treated primary cultures of bovine aortic endothelial cells with PPAR-delta specific ligand, GW501516 (50-800 nM) for 6 h.
RESULTS
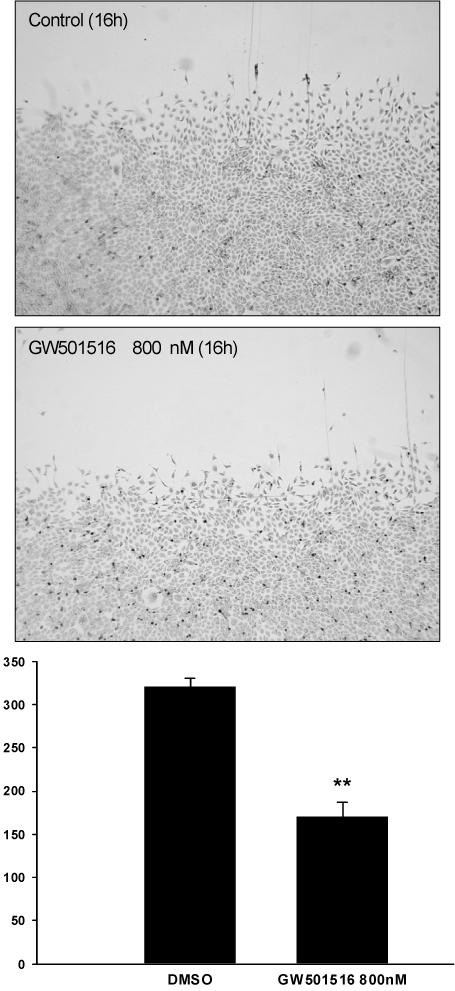
GW501516 dose-dependently decreased nitric oxide production without alteration in endothelial nitric oxide synthase (eNOS) expression. Analysis with phospho-specific antibodies against eNOS demonstrated that GW501516 significantly decreased the phosphorylation of eNOS at Serine1179 (eNOS-Ser1179). Concurrently, GW501516 also decreased the Akt phosphorylation. GW501516 did not affect endothelial cell proliferation or induce apoptosis. However, GW501516 inhibited endothelial cell migration, and tube formation in a high nanomolar concentration. The inhibition of endothelial cell tube formation by GW501516 was prevented by addition of the nitric oxide donor, DETA NONOate (5 microM). GW501516 was also found to inhibit angiogenesis in vivo in the chicken chorioallantoic membrane assay.
CONCLUSION
These results provide that high nanomolar range of GW501516 inhibits angiogenesis by a mechanism involving dephosphorylation of eNOS-Ser1179.
Keyword
MeSH Terms
-
Adipose Tissue
Animals
Antibodies, Phospho-Specific
Apoptosis
Atherosclerosis
Brain
Chickens
Chorioallantoic Membrane
DEET
Endothelial Cells
Humans
Ligands
Mice
Nitric Oxide
Nitric Oxide Synthase Type III
Nitroso Compounds
Peroxisomes
Phosphorylation
PPAR delta
Thiazoles
Tissue Donors
Antibodies, Phospho-Specific
DEET
Ligands
Nitric Oxide
Nitric Oxide Synthase Type III
Nitroso Compounds
PPAR delta
Thiazoles
Figure
Reference
-
1. Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997; 277:48–50.
Article2. Cooke JP. NO and angiogenesis. Atheroscler Suppl. 2003; 4:53–60.
Article3. Sivakumar B, Harry LE, Paleolog EM. Modulating angiogenesis: more vs less. JAMA. 2004; 292:972–977.4. van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA. 2005; 293:1509–1513.5. Varet J, Vincent L, Mirshahi P, Pille JV, Legrand E, Opolon P, Mishal Z, Soria J, Li H, Soria C. Fenofibrate inhibits angiogenesis in vitro and in vivo. Cell Mol Life Sci. 2003; 60:810–819.6. Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999; 274:9116–9121.
Article7. Vosper H, Patel L, Graham TL, Khoudoli GA, Hill A, Macphee CH, Pinto I, Smith SA, Suckling KE, Wolf CR, Palmer CN. The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J Biol Chem. 2001; 276:44258–44265.
Article8. Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2002; 99:303–308.
Article9. Shi Y, Hon M, Evans RM. The peroxisome proliferator-activated receptor delta, an integrator of transcriptional repression and nuclear receptor signaling. Proc Natl Acad Sci U S A. 2002; 99:2613–2618.
Article10. Michalik L, Desvergne B, Tan NS, Basu-Modak S, Escher P, Rieusset J, Peters JM, Kaya G, Gonzalez FJ, Zakany J, Metzger D, Chambon P, Duboule D, Wahli W. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)alpha and PPARbeta mutant mice. J Cell Biol. 2001; 154:799–814.
Article11. Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol. 2000; 20:5119–5128.
Article12. Kim HP, Lee JY, Jeong JK, Bae SW, Lee HK, Jo I. Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor alpha localized in caveolae. Biochem Biophys Res Commun. 1999; 263:257–262.13. Cho DH, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) gamma-dependent and PPARgamma-independent signaling pathways. J Biol Chem. 2004; 279:2499–2506.
Article14. van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, Isner JM. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997; 95:1030–1037.
Article15. Kim J, Kim KS, Shinn JW, Oh YS, Kim HT, Jo I, Shinn SH. The effect of antioxidants on glycated albumin-induced cytotoxicity in bovine retinal pericytes. Biochem Biophys Res Commun. 2002; 292:1010–1016.
Article16. Goodman SL, Vollmers HP, Birchmeier W. Control of cell locomotion: perturbation with an antibody directed against specific glycoproteins. Cell. 1985; 41:1029–1038.
Article17. Malinda KM, Nomizu M, Chung M, Delgado M, Kuratomi Y, Yamada Y, Kleinman HK, Ponce ML. Identification of laminin alpha1 and beta1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. FASEB J. 1999; 13:53–62.
Article18. Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992; 67:519–528.19. Crum R, Szabo S, Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985; 230:1375–1378.
Article20. Fleming I, Bauersachs J, Fisslthaler B, Busse R. Ca2+-independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ Res. 1998; 82:686–695.
Article21. Grimaldi PA. The roles of PPARs in adipocyte differentiation. Prog Lipid Res. 2001; 40:269–281.
Article22. Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999; 13:1561–1574.
Article23. Tan NS, Michalik L, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor (PPAR)-beta as a target for wound healing drugs: what is possible? Am J Clin Dermatol. 2003; 4:523–530.
Article24. Poirier H, Niot I, Monnot MC, Braissant O, Meunier-Durmort C, Costet P, Pineau T, Wahli W, Willson TM, Besnard P. Differential involvement of peroxisome-proliferator-activated receptors alpha and delta in fibrate and fatty-acid-mediated inductions of the gene encoding liver fatty-acid-binding protein in the liver and the small intestine. Biochem J. 2001; 355:481–488.
Article25. Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004; 2:e294.26. Oliver WR Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001; 98:5306–5311.
Article27. Ziche M, Morbidelli L, Masini E, Granger H, Geppetti P, Ledda F. Nitric oxide promotes DNA synthesis and cyclic GMP formation in endothelial cells from postcapillary venules. Biochem Biophys Res Commun. 1993; 192:1198–1203.
Article28. Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997; 100:3131–3139.
Article29. Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998; 101:731–736.
Article30. Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998; 101:2567–2578.
Article31. Hattori MA, Kato Y, Fujihara N. Retinoic acid suppression of endothelial nitric oxide synthase in porcine oocyte. Can J Physiol Pharmacol. 2002; 80:777–782.
Article32. Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003; 285:C499–C508.
Article33. Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003; 284:R1–R12.34. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999; 399:597–601.
Article35. Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001; 88:E68–E75.36. Taylor S, Folkman J. Protamine is an inhibitor of angiogenesis. Nature. 1982; 297:307–312.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Peroxisome Proliferator-Activated Receptor (PPAR) Delta on the Growth and Invasion of a Thyroid Cancer Cell Line
- Telmisartan Inhibits Nitric Oxide Production and Vessel Relaxation via Protein Phosphatase 2A-mediated Endothelial NO Synthase-Ser¹¹â·â¹ Dephosphorylation
- Peroxisome Proliferator Activated Receptor-delta (PPAR-delta)
- Peroxisome Proliferator-Activated Receptor Gamma(PPAR-gamma) Agonist Improves Endothelial Function in Diabetic Patients with Metabolic Syndrome: Pivotal Role of NOx and Inflammation
- Peroxisome Proliferator-activated Receptors (PPARs) in Diabetic Nephropathy