J Korean Soc Hypertens.
2011 Jun;17(2):57-64. 10.5646/jksh.2011.17.2.57.
Expression of Lectin like Oxidized Low Density Lipoprotein Receptor-1 in the Spontaneous Hypertensive Rat with High Cholesterol Diet
- Affiliations
-
- 1Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea. kiseok@jejunu.ac.kr
- 2Department of Internal Medicine, Chungbuk National University School of Medicine, Cheongju, Korea.
- KMID: 2196456
- DOI: http://doi.org/10.5646/jksh.2011.17.2.57
Abstract
- BACKGROUND
Lectin-like, oxidized, low-density lipoprotein receptorreceptors (LOX-1) recognizes recognize vascular oxidized low-density lipoprotein (LDL), which may play an important role in the pathogenesis of atherosclerosis. We investigated the expressions expression of LOX-1 and redox-regulating thyoredoxinthioredoxin systems in a hypertension and hypercholesterolemia rat model.
METHODS
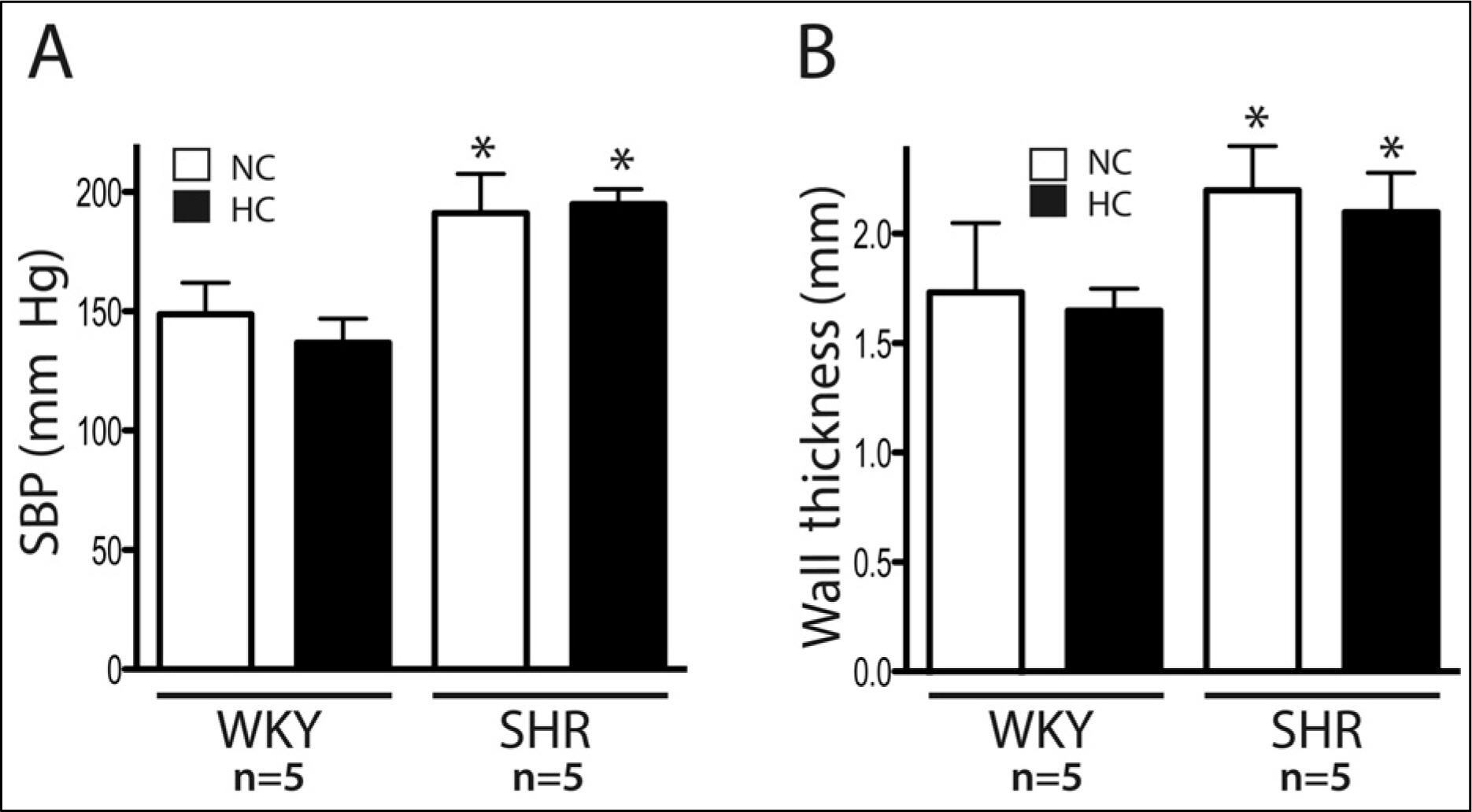
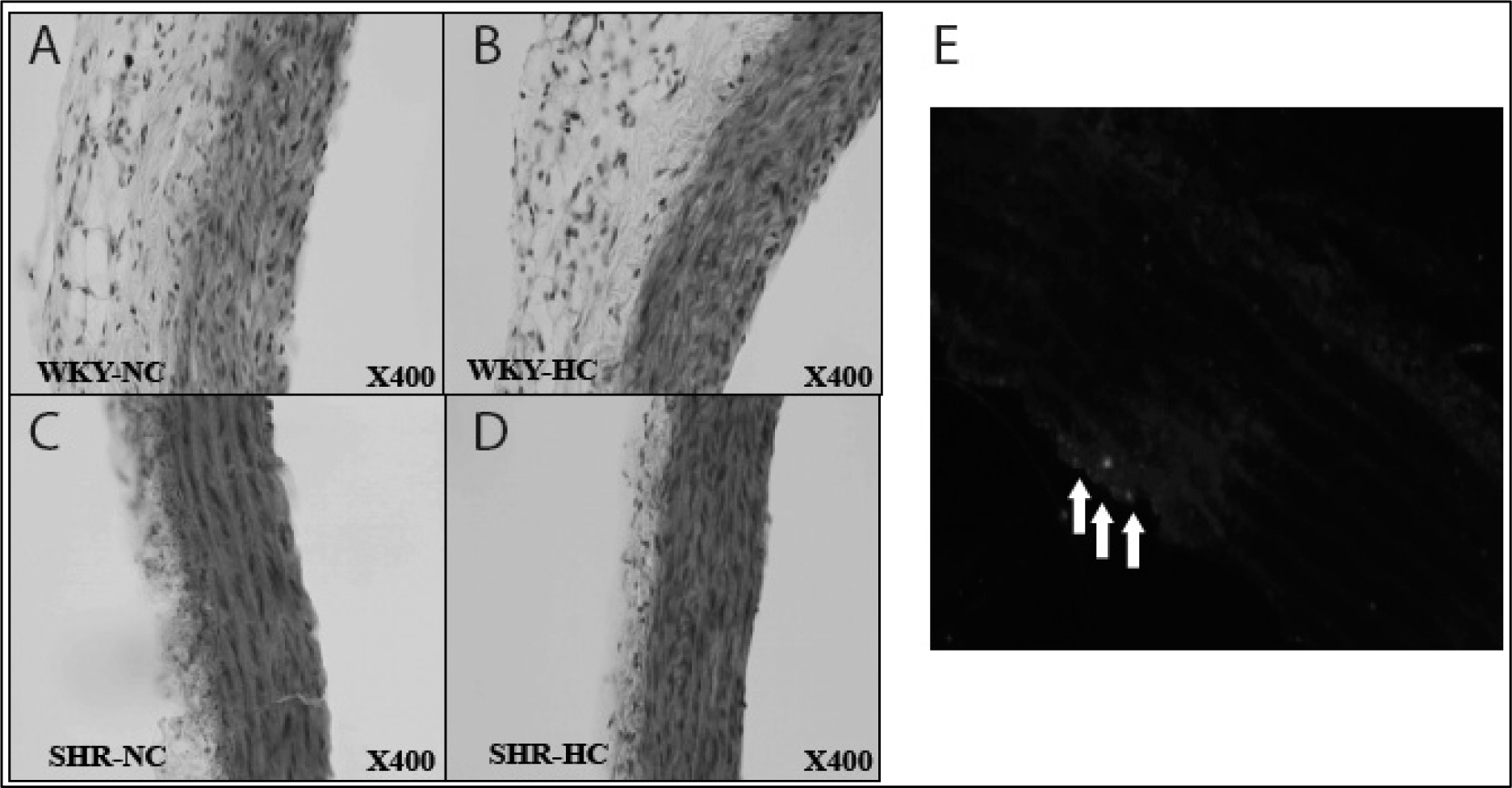
Spontaneously hypertensive rats (SHR) and Wistar-Kyoto rat (WKY) rats were fed with a normal cholesterol diet (NC) and a high cholesterol diet (HC) for 4 weeks. Plasma LDL cholesterol levels and blood pressure were measured at 1 and 4 weeks. Histological changes of atherosclerosis in the vessel was evaluated by hematoxylin and eosin staining and immunocytochemistry. The expressions expression of LOX-1 and thyoredoixnthioredoxin were measured by Western western blot analysis.
RESULTS
In the SHR groupsgroup, blood pressure after 4 weeks was significantly higher than initial levels. LDL-cholesterol levels in the SHR-HC group were increased at 4 weeks (15.3 +/- 2.6 mg/dL vs. 20.2 +/- 2.6 mg/dL, p < 0.01) compared with the SHR-NC group. In oxyblot analysis, the degree of oxidative stress of in the SHR-HC group was significantly higher than in the SHR-NC group (p < 0.05). The expressions expression of LOX-1 and Trx were was significantly increased in the SHR-HC group compared with the SHR-NC group (p < 0.05) on western blot analysis. Focal overexpressions overexpression of LOX-1 were was observed at the intima layer of the thoracic aorta, and was which wereonly observed in the SHR-HC group.
CONCLUSIONS
The expressions expression of LOX-1 and oxidative stress were was significantly increased in the "hypertension with hypercholesterol" rat model. These findings suggested suggest that LOX-1 and redox systems may play a certain role in development and progression of atherosclerosis.
Keyword
MeSH Terms
-
Animals
Aorta, Thoracic
Atherosclerosis
Blood Pressure
Blotting, Western
Cholesterol
Cholesterol, LDL
Diet
Eosine Yellowish-(YS)
Glycosaminoglycans
Hematoxylin
Hypercholesterolemia
Hypertension
Immunohistochemistry
Lipoproteins
Lipoproteins, LDL
Oxidation-Reduction
Oxidative Stress
Plasma
Rats
Rats, Inbred SHR
Cholesterol
Cholesterol, LDL
Eosine Yellowish-(YS)
Glycosaminoglycans
Hematoxylin
Lipoproteins
Lipoproteins, LDL
Figure
Reference
-
References
1. Ogura S, Kakino A, Sato Y, Fujita Y, Iwamoto S, Otsui K, et al. Lox-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ J. 2009; 73:1993–9.
Article2. Kang BY, Hu C, Prayaga S, Khaidakov M, Sawamura T, Seung KB, et al. LOX-1 dependent overexpression of immunoglobulins in cardiomyocytes in response to angiotensin II. Biochem Biophys Res Commun. 2009; 379:395–9.
Article3. Navarra T, Del Turco S, Berti S, Basta G. The lectin-like oxidized low-density lipoprotein receptor-1 and its soluble form: cardiovascular implications. J Atheroscler Thromb. 2010; 17:317–31.
Article4. Kume N, Kita T. Apoptosis of vascular cells by oxidized LDL: involvement of caspases and LOX-1 and its implication in atherosclerotic plaque rupture. Circ Res. 2004; 94:269–70.5. Koharyova M, Kolarova M. Oxidative stress and thioredoxin system. Gen Physiol Biophys. 2008; 27:71–84.6. Hofnagel O, Luechtenborg B, Stolle K, Lorkowski S, Eschert H, Plenz G, et al. Proinflammatory cytokines regulate LOX-1 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004; 24:1789–95.
Article7. Li DY, Chen HJ, Staples ED, Ozaki K, Annex B, Singh BK, et al. Oxidized low-density lipoprotein receptor LOX-1 and apoptosis in human atherosclerotic lesions. J Cardiovasc Pharmacol Ther. 2002; 7:147–53.
Article8. Nowicki M, Muller K, Serke H, Kosacka J, Vilser C, Ricken A, et al. Oxidized low-density lipoprotein (oxLDL)-induced cell death in dorsal root ganglion cell cultures depends not on the lectin-like oxLDL receptor-1 but on the toll-like receptor-4. J Neurosci Res. 2010; 88:403–12.9. Tanigawa H, Miura S, Zhang B, Uehara Y, Matsuo Y, Fujino M, et al. Low-density lipoprotein oxidized to various degrees activates ERK1/2 through Lox-1. Atherosclerosis. 2006; 188:245–50.
Article10. Kanata S, Akagi M, Nishimura S, Hayakawa S, Yoshida K, Sawamura T, et al. Oxidized LDL binding to LOX-1 upre-gulates VEGF expression in cultured bovine chondrocytes through activation of PPAR-gamma. Biochem Biophys Res Commun. 2006; 348:1003–10.11. Fujita Y, Kakino A, Nishimichi N, Yamaguchi S, Sato Y, Machida S, et al. Oxidized LDL receptor LOX-1 binds to C-reactive protein and mediates its vascular effects. Clin Chem. 2009; 55:285–94.
Article12. Li D, Mehta JL. Intracellular signaling of LOX-1 in endothelial cell apoptosis. Circ Res. 2009; 104:566–8.
Article13. Takaya T, Wada H, Morimoto T, Sunagawa Y, Kawamura T, Takanabe-Mori R, et al. Left ventricular expression of lectin-like oxidized low-density lipoprotein receptor-1 in failing rat hearts. Circ J. 2010; 74:723–9.
Article14. Chen J, Hui ST, Couto FM, Mungrue IN, Davis DB, Attie AD, et al. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008; 22:3581–94.
Article15. Adluri RS, Thirunavukkarasu M, Zhan L, Akita Y, Samuel SM, Otani H, et al. Thioredoxin 1 enhances neo-vascularization and reduces ventricular remodeling during chronic myocardial infarction: a study using thioredoxin 1 transgenic mice. J Mol Cell Cardiol. 2011; 50:239–47.
Article16. Soejima H, Suefuji H, Miyamoto S, Kajiwaram I, Kojima S, Hokamaki J, et al. Increased plasma thioredoxin in patients with acute myocardial infarction. Clin Cardiol. 2003; 26:583–7.
Article17. Malik G, Gorbounov N, Das S, Gurusamy N, Otani H, Maulik N, et al. Ischemic preconditioning triggers nuclear translocation of thioredoxin and its interaction with Ref-1 potentiating a survival signal through the PI-3-kinase-Akt pathway. Antioxid Redox Signal. 2006; 8:2101–9.
Article18. Martinez-Pinna R, Lindholt JS, Blanco-Colio LM, Dejouvencel T, Madrigal-Matute J, Ramos-Mozo P, et al. Increased levels of thioredoxin in patients with abdominal aortic aneurysms (AAAs). A potential link of oxidative stress with AAA evolution. Atherosclerosis. 2010; 212:333–8.
Article19. Park KJ, Kim YJ, Choi EJ, Park NK, Kim GH, Kim SM, et al. Expression pattern of the thioredoxin system in human endothelial progenitor cells and endothelial cells under hypoxic injury. Korean Circ J. 2010; 40:651–8.
Article20. Chinellato A, Ragazzi E, Petrelli L, Paro M, Mironov A, Aliev G. Effect of cholesterol-supplemented diet in heritable hyperlipidemic Yoshida rats: functional and morphological characterization of thoracic aorta. Atherosclerosis. 1994; 106:51–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Soluble Lectin-Like Oxidized Low-Density Lipoprotein Receptor 1 Is Inversely Correlated with the Activity of ANCA-Associated Vasculitis
- The effects of Brassica juncea L. leaf extract on obesity and lipid profiles of rats fed a high-fat/high-cholesterol diet
- Hypolipidemic Effects and Safety of Lovastatin in Patients with Primary Hypercholesterolemia
- Recombinant Human Thioredoxin-1 Protects Macrophages from Oxidized Low-Density Lipoprotein-Induced Foam Cell Formation and Cell Apoptosis
- Low-Carbohydrate Diets in Korea: Why Does It Matter, and What Is Next?