The Korean guideline for colorectal cancer screening
- Affiliations
-
- 1Center for Colorectal Cancer, National Cancer Center, Goyang, Korea.
- 2Department of Radiology, National Cancer Center, Goyang, Korea.
- 3Department of Laboratory Medicine, Catholic Kwandong University College of Medicine, Incheon, Korea.
- 4National Cancer Control Institute, National Cancer Center, Goyang, Korea.
- 5Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 6Center for Preventive Medicine and Public Health, Seoul National University Bundang Hospital, Seongnam, Korea.
- 7Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 8Department of Surgery, The Catholic University of Korea Saint Vincent's Hospital, Suwon, Korea.
- 9Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 10Department of Pathology, Korea University College of Medicine, Seoul, Korea.
- 11Department of Radiology, Hanyang University College of Medicine, Seoul, Korea.
- 12Department of Internal Medicine, Chungang University College of Medicine, Seoul, Korea.
- 13Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 14Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 15Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 16Department of Surgery, Korea University Ansan Hospital, Ansan, Korea.
- 17Department of Radiology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 18Department of Family Medicine/Health Promotion Center, KEPCO Medical Center, Seoul, Korea.
- 19Department of Family Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 20Department of Preventive Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 21Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. syjeong@snu.ac.kr
- KMID: 2195038
- DOI: http://doi.org/10.5124/jkma.2015.58.5.420
Abstract
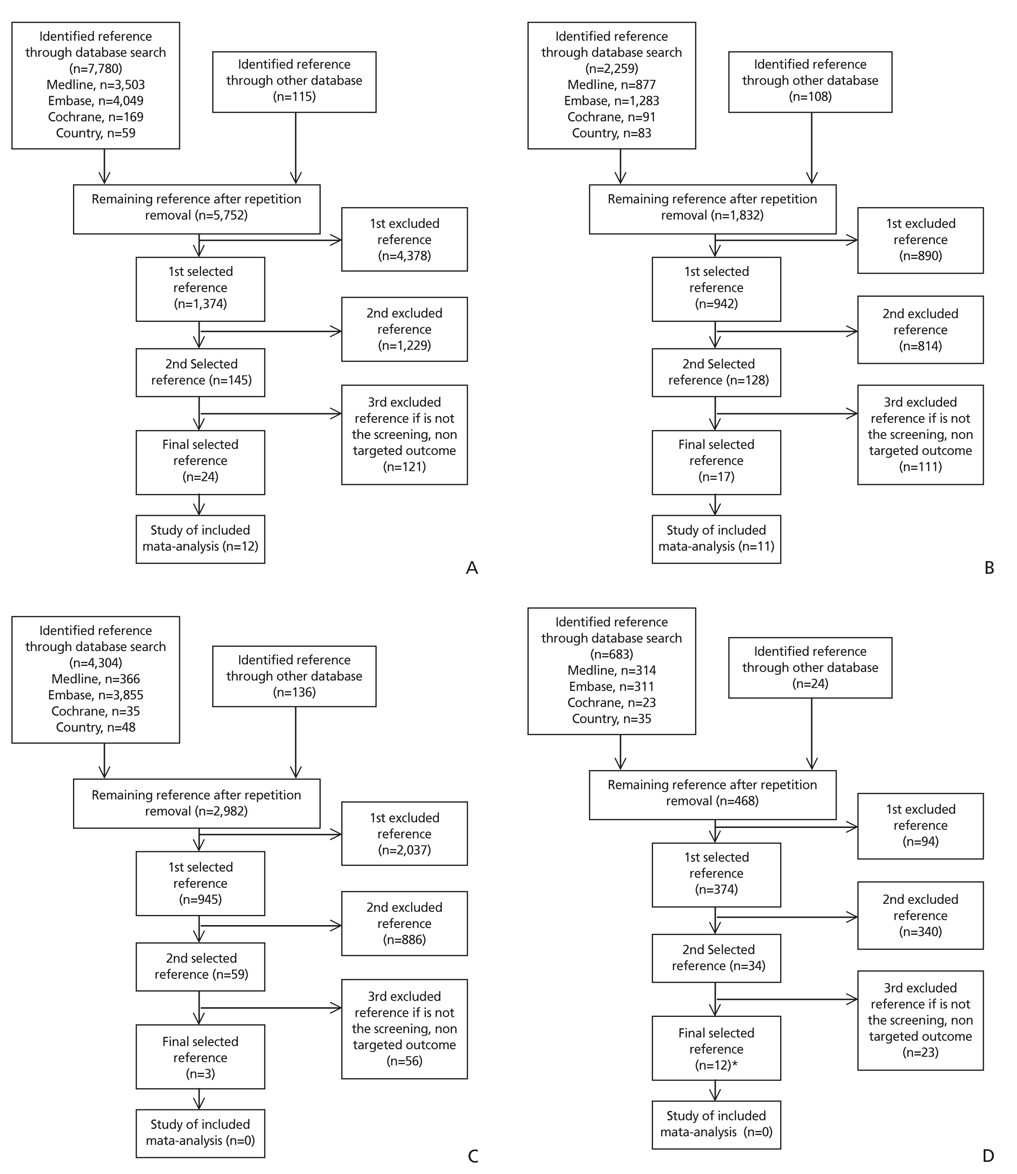
- Colorectal cancer is the third most common cancer in Korea; it is the second most common cancer in men and the third most common in women. The incidence rate in Korea has continuously increased since 1999 when the National Cancer Registry statistics began. Currently; there are several screening modalities; that have been recommended by expert societies, including fecal occult blood test, colonoscopy, computed tomographic colonography The annual fecal immunochemical test (FIT) has been used in adults aged 50 and older as part of the National Cancer Screening Program in Korea since 2004. Although several study results from regional or national colorectal cancer screening programs in other countries have been reported, the National Cancer Screening Program in Korea has not yet been evaluated with evidence-based methods. Herein report the consensus statements on the National Screening Guideline for colorectal cancer developed by a multi-society expert committee in Korea, as follows: 1) We recommend annual or biennial FIT for screening for colorectal cancer in asymptomatic adults, beginning at 45 years of age and continuing until 80 years (recommendation B). 2) There is no evidence for the risks or benefits of FIT in adults older than 80 years (recommendation I). 3) Selective use of colonoscopy for colorectal cancer screening is recommended, taking into consideration individual preference and the risk of colorectal cancer (recommendation C). 4) There is no evidence for the risks or benefits of double-contrast barium enema for colorectal cancer screening in asymptomatic adults (recommendation I). 5) There is no evidence for the risks or benefits of computed tomographic colonography for colorectal cancer screening in asymptomatic adults (recommendation I).
Keyword
MeSH Terms
Figure
Cited by 7 articles
-
Quality management of medical imaging for public health screening
Woo Kyoung Jeong, Eun Hye Lee, Seung Eun Jung
J Korean Med Assoc. 2015;58(12):1125-1131. doi: 10.5124/jkma.2015.58.12.1125.Management of long-term colorectal cancer survivors in Korea
Hee-Taik Kang, Hyun Jung Bahk, Jae-Yong Shim, Nam Kyu Kim
J Korean Med Assoc. 2016;59(4):276-286. doi: 10.5124/jkma.2016.59.4.276.Risk of Diabetes in Subjects with Positive Fecal Immunochemical Test: A Nationwide Population-Based Study
Kwang Woo Kim, Hyun Jung Lee, Kyungdo Han, Jung Min Moon, Seung Wook Hong, Eun Ae Kang, Jooyoung Lee, Hosim Soh, Seong-Joon Koh, Jong Pil Im, Joo Sung Kim
Endocrinol Metab. 2021;36(5):1069-1077. doi: 10.3803/EnM.2021.1119.Update on Diagnosis and Treatment of Colorectal Cancer
Chan Wook Kim
Ewha Med J. 2022;45(4):e8. doi: 10.12771/emj.2022.e8.Overview of the National Cancer Screening Program for Colorectal Cancer in Korea over 14 Years (2004–2017)
Bomi Park, Eun Young Her, Kyeongmin Lee, Fatima Nari, Jae Kwan Jun, Kui Son Choi, Mina Suh
Cancer Res Treat. 2023;55(3):910-917. doi: 10.4143/crt.2022.1432.National cancer screening program for colorectal cancer in Korea
Seung Min Baik, Ryung-Ah Lee
Ann Surg Treat Res. 2023;105(6):333-340. doi: 10.4174/astr.2023.105.6.333.Cost-Utility Analysis for Colorectal Cancer Screening According to the Initiating Age of National Cancer Screening Program in Korea
Seowoo Bae, Kyeongmin Lee, Byung Chang Kim, Jae Kwan Jun, Kui Son Choi, Mina Suh
J Korean Med Sci. 2024;39(10):e98. doi: 10.3346/jkms.2024.39.e98.
Reference
-
1. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, Lee KH. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015; 47:127–141.
Article2. Statistics Korea. Cause of statistics in 2013 [Internet]. Daejeon: Statistics Korea;2013. cited 2015 Apr 22. Available from: http://www.kostat.go.kr.3. Shin A, Jung KW, Won YJ. Colorectal cancer mortality in Hong Kong of China, Japan, South Korea, and Singapore. World J Gastroenterol. 2013; 19:979–983.
Article4. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008; 149:627–637.5. Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, Pickhardt P, Rex DK, Thorson A, Winawer SJ. American Cancer Society Colorectal Cancer Advisory Group. US Multi-Society Task Force. American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008; 58:130–160.
Article6. Scottish Intercollegiate Guidelines Network. Scottish Cancer Therapy Network. Diagnosis and management of colorectal cancer: a national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network;2011.7. Lee BI, Hong SP, Kim SE, Kim SH, Kim HS, Hong SN, Yang DH, Shin SJ, Lee SH, Park DI, Kim YH, Kim HJ, Yang SK, Kim HJ, Jeon HJ. Multi-Society Task Force for Development of Guidelines for Colorectal Polyp Screening, Surveillance and Management. Korean guidelines for colorectal cancer screening and polyp detection. Clin Endosc. 2012; 45:25–43.
Article8. European Colorectal Cancer Screening Guidelines Working Group. von Karsa L, Patnick J, Segnan N, Atkin W, Halloran S, Lansdorp-Vogelaar I, Malila N, Minozzi S, Moss S, Quirke P, Steele RJ, Vieth M, Aabakken L, Altenhofen L, Ancelle-Park R, Antoljak N, Anttila A, Armaroli P, Arrossi S, Austoker J, Banzi R, Bellisario C, Blom J, Brenner H, Bretthauer M, Camargo Cancela M, Costamagna G, Cuzick J, Dai M, Daniel J, Dekker E, Delicata N, Ducarroz S, Erfkamp H, Espinàs JA, Faivre J, Faulds Wood L, Flugelman A, Frkovic-Grazio S, Geller B, Giordano L, Grazzini G, Green J, Hamashima C, Herrmann C, Hewitson P, Hoff G, Holten I, Jover R, Kaminski MF, Kuipers EJ, Kurtinaitis J, Lambert R, Launoy G, Lee W, Leicester R, Leja M, Lieberman D, Lignini T, Lucas E, Lynge E, Madai S, Marinho J, Maucec Zakotnik J, Minoli G, Monk C, Morais A, Muwonge R, Nadel M, Neamtiu L, Peris Tuser M, Pignone M, Pox C, Primic-Zakelj M, Psaila J, Rabeneck L, Ransohoff D, Rasmussen M, Regula J, Ren J, Rennert G, Rey J, Riddell RH, Risio M, Rodrigues V, Saito H, Sauvaget C, Scharpantgen A, Schmiegel W, Senore C, Siddiqi M, Sighoko D, Smith R, Smith S, Suchanek S, Suonio E, Tong W, Törnberg S, Van Cutsem E, Vignatelli L, Villain P, Voti L, Watanabe H, Watson J, Winawer S, Young G, Zaksas V, Zappa M, Valori R. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013; 45:51–59.9. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009; 150:1–8.
Article10. Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009; 7:770–775.
Article11. Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest Endosc. 2012; 76:110–117.
Article12. Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, Imamura Y, Willett WC, Rosner BA, Fuchs CS, Giovannucci E, Ogino S, Chan AT. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013; 369:1095–1105.
Article13. Brenner H, Chang-Claude J, Seiler CM, Sturmer T, Hoffmeister M. Does a negative screening colonoscopy ever need to be repeated? Gut. 2006; 55:1145–1150.
Article14. Cotterchio M, Manno M, Klar N, McLaughlin J, Gallinger S. Colorectal screening is associated with reduced colorectal cancer risk: a case-control study within the population-based Ontario Familial Colorectal Cancer Registry. Cancer Causes Control. 2005; 16:865–875.
Article15. Singh H, Nugent Z, Mahmud SM, Demers AA, Bernstein CN. Predictors of colorectal cancer after negative colonoscopy: a population-based study. Am J Gastroenterol. 2010; 105:663–673.
Article16. Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M. Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001; 48:812–815.
Article17. Singh H, Turner D, Xue L, Targownik LE, Bernstein CN. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA. 2006; 295:2366–2373.
Article18. Doubeni CA, Weinmann S, Adams K, Kamineni A, Buist DS, Ash AS, Rutter CM, Doria-Rose VP, Corley DA, Greenlee RT, Chubak J, Williams A, Kroll-Desrosiers AR, Johnson E, Webster J, Richert-Boe K, Levin TR, Fletcher RH, Weiss NS. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested casecontrol study. Ann Intern Med. 2013; 158(5 Pt 1):312–320.
Article19. Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006; 355:1863–1872.
Article20. Pox CP, Altenhofen L, Brenner H, Theilmeier A, Von Stillfried D, Schmiegel W. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012; 142:1460–1467.
Article21. Senore C, Ederle A, Fantin A, Andreoni B, Bisanti L, Grazzini G, Zappa M, Ferrero F, Marutti A, Giuliani O, Armaroli P, Segnan N. Acceptability and side-effects of colonoscopy and sigmoidoscopy in a screening setting. J Med Screen. 2011; 18:128–134.
Article22. Rutter CM, Johnson E, Miglioretti DL, Mandelson MT, Inadomi J, Buist DS. Adverse events after screening and followup colonoscopy. Cancer Causes Control. 2012; 23:289–296.
Article23. Khalid-de Bakker CA, Jonkers DM, Hameeteman W, de Ridder RJ, Masclee AA, Stockbrugger RW. Cardiopulmonary events during primary colonoscopy screening in an average risk population. Neth J Med. 2011; 69:186–191.24. Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, Schulman J. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006; 145:880–886.
Article25. Lee YC, Wang HP, Chiu HM, Lin CP, Huang SP, Lai YP, Wu MS, Chen MF, Lin JT. Factors determining post-colonoscopy abdominal pain: prospective study of screening colonoscopy in 1000 subjects. J Gastroenterol Hepatol. 2006; 21:1575–1580.
Article26. Nelson DB, McQuaid KR, Bond JH, Lieberman DA, Weiss DG, Johnston TK. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc. 2002; 55:307–314.
Article27. Zubarik R, Ganguly E, Benway D, Ferrentino N, Moses P, Vecchio J. Procedure-related abdominal discomfort in patients undergoing colorectal cancer screening: a comparison of colonoscopy and flexible sigmoidoscopy. Am J Gastroenterol. 2002; 97:3056–3061.
Article28. Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Interval cancers after negative colonoscopy: population-based casecontrol study. Gut. 2012; 61:1576–1582.
Article29. Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol. 2006; 4:1259–1264.
Article30. Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007; 132:96–102.
Article31. Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Minnesota Colon Cancer Control Study. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med. 1993; 328:1365–1371.
Article32. Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996; 348:1467–1471.
Article33. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999; 91:434–437.
Article34. Jorgensen OD, Kronborg O, Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002; 50:29–32.
Article35. Scholefield JH, Moss S, Sufi F, Mangham CM, Hardcastle JD. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut. 2002; 50:840–844.
Article36. Kronborg O, Jorgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol. 2004; 39:846–851.
Article37. Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008; 95:1029–1036.
Article38. Whynes DK, Mangham CM, Balfour TW, Scholefield JH. Analysis of deaths occurring within the Nottingham trial of faecal occult blood screening for colorectal cancer. Gut. 2010; 59:1088–1093.
Article39. Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, Snover DC, Schuman LM. The effect of fecal occultblood screening on the incidence of colorectal cancer. N Engl J Med. 2000; 343:1603–1607.
Article40. Kewenter J, Brevinge H, Engaras B, Haglind E, Ahren C. Results of screening, rescreening, and follow-up in a prospective randomized study for detection of colorectal cancer by fecal occult blood testing. Results for 68,308 subjects. Scand J Gastroenterol. 1994; 29:468–473.
Article41. Paimela H, Malila N, Palva T, Hakulinen T, Vertio H, Jarvinen H. Early detection of colorectal cancer with faecal occult blood test screening. Br J Surg. 2010; 97:1567–1571.
Article42. Nakama H, Kamijo N, Abdul Fattah AS, Zhang B. Validity of immunological faecal occult blood screening for colorectal cancer: a follow up study. J Med Screen. 1996; 3:63–65.
Article43. Liu HH, Huang TW, Chen HL, Wang TH, Lin JT. Clinicopathologic significance of immunohistochemical fecal occult blood test in subjects receiving bidirectional endoscopy. Hepatogastroenterology. 2003; 50:1390–1392.44. Nakazato M, Yamano H, Matsushita H, Sato K, Fujita K, Yamanaka Y, Imai Y. Immunologic fecal occult blood test for colorectal cancer screening. Japan Med Assoc J. 2006; 49:203–207.45. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Sakaguchi K, Shiratori Y. Sensitivity of immunochemical fecal occult blood test to small colorectal adenomas. Am J Gastroenterol. 2007; 102:2259–2264.
Article46. Chiu HM, Lee YC, Tu CH, Chen CC, Tseng PH, Liang JT, Shun CT, Lin JT, Wu MS. Association between early stage colon neoplasms and false-negative results from the fecal immunochemical test. Clin Gastroenterol Hepatol. 2013; 11:832–838.
Article47. Kung JW, Levine MS, Glick SN, Lakhani P, Rubesin SE, Laufer I. Colorectal cancer: screening double-contrast barium enema examination in average-risk adults older than 50 years. Radiology. 2006; 240:725–735.
Article48. Blakeborough A, Sheridan MB, Chapman AH. Complications of barium enema examinations: a survey of UK Consultant Radiologists 1992 to 1994. Clin Radiol. 1997; 52:142–148.
Article49. Neri E, Faggioni L, Cerri F, Turini F, Angeli S, Cini L, Perrone F, Paolicchi F, Bartolozzi C. CT colonography versus doublecontrast barium enema for screening of colorectal cancer: comparison of radiation burden. Abdom Imaging. 2010; 35:596–601.
Article50. Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003; 349:2191–2200.
Article51. Vogt C, Cohnen M, Beck A, vom Dahl S, Aurich V, Modder U, Haussinger D. Detection of colorectal polyps by multislice CT colonography with ultra-low-dose technique: comparison with high-resolution videocolonoscopy. Gastrointest Endosc. 2004; 60:201–209.
Article52. Johnson CD, Fletcher JG, MacCarty RL, Mandrekar JN, Harmsen WS, Limburg PJ, Wilson LA. Effect of slice thickness and primary 2D versus 3D virtual dissection on colorectal lesion detection at CT colonography in 452 asymptomatic adults. AJR Am J Roentgenol. 2007; 189:672–680.
Article53. Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, Menias CO, Siewert B, Cheema JI, Obregon RG, Fidler JL, Zimmerman P, Horton KM, Coakley K, Iyer RB, Hara AK, Halvorsen RA Jr, Casola G, Yee J, Herman BA, Burgart LJ, Limburg PJ. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008; 359:1207–1217.
Article54. Kim YS, Kim N, Kim SH, Park MJ, Lim SH, Yim JY, Cho KR, Kim SS, Kim DH, Eun HW, Cho KS, Kim JH, Choi BI, Jung HC, Song IS, Shin CS, Cho SH, Oh BH. The efficacy of intravenous contrast-enhanced 16-raw multidetector CT colonography for detecting patients with colorectal polyps in an asymptomatic population in Korea. J Clin Gastroenterol. 2008; 42:791–798.
Article55. Graser A, Stieber P, Nagel D, Schafer C, Horst D, Becker CR, Nikolaou K, Lottes A, Geisbusch S, Kramer H, Wagner AC, Diepolder H, Schirra J, Roth HJ, Seidel D, Goke B, Reiser MF, Kolligs FT. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009; 58:241–248.
Article56. Sakamoto T, Mitsuzaki K, Utsunomiya D, Matsuda K, Yamamura S, Urata J, Kawakami M, Yamashita Y. Detection of flat colorectal polyps at screening CT colonography in comparison with conventional polypoid lesions. Acta Radiol. 2012; 53:714–719.
Article57. Zalis ME, Blake MA, Cai W, Hahn PF, Halpern EF, Kazam IG, Keroack M, Magee C, Nappi JJ, Perez-Johnston R, Saltzman JR, Vij A, Yee J, Yoshida H. Diagnostic accuracy of laxativefree computed tomographic colonography for detection of adenomatous polyps in asymptomatic adults: a prospective evaluation. Ann Intern Med. 2012; 156:692–702.
Article58. Lefere P, Silva C, Gryspeerdt S, Rodrigues A, Vasconcelos R, Teixeira R, de Gouveia FH. Teleradiology based CT colonography to screen a population group of a remote island; at average risk for colorectal cancer. Eur J Radiol. 2013; 82:e262–e267.
Article59. Sosna J, Blachar A, Amitai M, Barmeir E, Peled N, Goldberg SN, Bar-Ziv J. Colonic perforation at CT colonography: assessment of risk in a multicenter large cohort. Radiology. 2006; 239:457–463.
Article60. Liedenbaum MH, Venema HW, Stoker J. Radiation dose in CT colonography: trends in time and differences bet-ween daily practice and screening protocols. Eur Radiol. 2008; 18:2222–2230.
Article61. Hoff G, Grotmol T, Skovlund E, Bretthauer M. Norwegian Colorectal Cancer Prevention Study Group. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009; 338:b1846.
Article62. Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J. UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010; 375:1624–1633.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening strategy for colorectal cancer according to risk
- Colon Cancer Screening—Is It Necessary to Start under the Age of 50?
- Colorectal Cancer Screening: Stool DNA and Other Noninvasive Modalities
- Colorectal Cancer Screening and Surveillance in the Elderly: Updates and Controversies
- The impact of COVID-19 on screening for colorectal, gastric, breast, and cervical cancer in Korea