J Korean Med Assoc.
2011 May;54(5):491-501. 10.5124/jkma.2011.54.5.491.
Stem cells in musculoskeletal system for clinical application
- Affiliations
-
- 1Department of Orthopedic Surgery, Ajou University School of Medicine, Ajou University Medical Center, Suwon, Korea. bhmin@ajou.ac.kr
- 2Cell Therapy Center, Ajou University Medical Center, Suwon, Korea.
- KMID: 2190708
- DOI: http://doi.org/10.5124/jkma.2011.54.5.491
Abstract
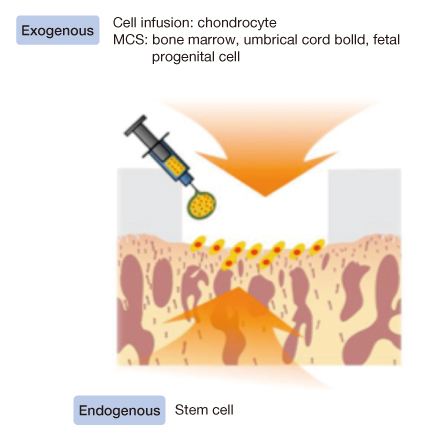
- Mesenchymal stem cells (MSCs) play a crucial role in the proliferation and differentiation of human tissue such as bone, cartilage, muscle, fat, and fibroblasts. Various surgical techniques have been developed for the repair of the musculoskeletal system, but they can be often limited. Thus, the efforts that can be employed in treatment for MSCs population of degenerative musculoskeletal diseases are underway. Patients who have a musculoskeletal disease with low numbers of functional MSCs will be treated using a focus on cell-based therapy. The ideal clinical application is to engineer material/scaffolds that are capable of delivering therapeutic cells that can regenerate and repair damaged tissue. The ability related to MSCs differentiation using biomaterial systems offers a minimally invasive therapeutic option for diseases of the musculoskelecal system and tissue repair. Understanding the natural mechanisms for this delivery is essential to the success of tissue engineering biomaterials that deliver therapeutic cells.
MeSH Terms
Figure
Cited by 1 articles
-
The principles of tissue engineering and its recent advances and future prospects
Woo Seob Kim
J Korean Med Assoc. 2014;57(2):145-154. doi: 10.5124/jkma.2014.57.2.145.
Reference
-
1. Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009. 20:537–544.
Article2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article3. Kuo CK, Li WJ, Mauck RL, Tuan RS. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006. 18:64–73.
Article4. Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009. 106:984–991.
Article5. Abarbanell AM, Coffey AC, Fehrenbacher JW, Beckman DJ, Herrmann JL, Weil B, Meldrum DR. Proinflammatory cytokine effects on mesenchymal stem cell therapy for the ischemic heart. Ann Thorac Surg. 2009. 88:1036–1043.
Article6. Haider HK, Jiang S, Idris NM, Ashraf M. IGF-1-overexpress-ing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008. 103:1300–1308.
Article7. Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009. 4:206–216.
Article8. Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003. 5:32–45.9. Kavalkovich KW, Boynton RE, Murphy JM, Barry F. Chon-drogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002. 38:457–466.
Article10. Radice M, Brun P, Cortivo R, Scapinelli R, Battaliard C, Aba-tangelo G. Hyaluronan-based biopolymers as delivery vehi-cles for bone-marrow-derived mesenchymal progenitors. J Biomed Mater Res. 2000. 50:101–109.
Article11. Nuttelman CR, Tripodi MC, Anseth KS. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A. 2004. 68:773–782.
Article12. Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003. 9:679–688.
Article13. Tiedeman JJ, Garvin KL, Kile TA, Connolly JF. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics. 1995. 18:1153–1158.
Article14. Gepstein R, Weiss RE, Hallel T. Bridging large defects in bone by demineralized bone matrix in the form of a powder. A ra-diographic, histological, and radioisotope-uptake study in rats. J Bone Joint Surg Am. 1987. 69:984–992.
Article15. Summers BN, Eisenstein SM. Donor site pain from the ilium: a complication of lumbar spine fusion. J Bone Joint Surg Br. 1989. 71:677–680.
Article16. Coventry MB, Tapper EM. Pelvic instability: a consequence of removing iliac bone for grafting. J Bone Joint Surg Am. 1972. 54:83–101.17. Lee SW, Padmanabhan P, Ray P, Gambhir SS, Doyle T, Contag C, Goodman SB, Biswal S. Stem cell-mediated accelerated bone healing observed with in vivo molecular and small animal imaging technologies in a model of skeletal injury. J Orthop Res. 2009. 27:295–302.
Article18. Granero-Molto F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP, Spagnoli A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009. 27:1887–1898.
Article19. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991. 9:641–650.
Article20. Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992. 13:69–80.
Article21. Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992. 13:81–88.
Article22. Beresford JN. Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Relat Res. 1989. (240):270–280.
Article23. Brighton CT. The biology of fracture repair. Instr Course Lect. 1984. 33:60–82.24. Bruder SP, Eames BF, Haynesworth SE. Osteogenic induction of purified human mesenchymal stem cells in vitro: quantitative assessment of the osteoblastic phenotype. Trans Orthop Res Soc. 1995. 20:464.25. Takahashi T, Tominaga T, Watabe N, Yokobori AT Jr, Sasada H, Yoshimoto T. Use of porous hydroxyapatite graft containing recombinant human bone morphogenetic protein-2 for cervical fusion in a caprine model. J Neurosurg. 1999. 90:2 Suppl. 224–230.
Article26. Asahina I, Watanabe M, Sakurai N, Mori M, Enomoto S. Repair of bone defect in primate mandible using a bone morphogenetic protein (BMP)-hydroxyapatite-collagen composite. J Med Dent Sci. 1997. 44:63–70.27. Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007. 13:642–648.
Article28. Beauchamp JR, Morgan JE, Pagel CN, Partridge TA. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999. 144:1113–1122.
Article29. Rossi CA, Elvassore N, Flaibani M, Pozzobon M, Blaauw B, Reggiani C. Freshly isolated mouse satellite cells embedded in hyaluronan-based hydrogel allow hystological and functional recovery of partially ablated tibialis anterioris muscle. In : International Society for Stem Cell Research 7th Annual Meeting; 2009 Jul 8-11; Barcelona, Spain.30. Kang SW, Bada LP, Kang CS, Lee JS, Kim CH, Park JH, Kim BS. Articular cartilage regeneration with microfracture and hyaluronic acid. Biotechnol Lett. 2008. 30:435–439.
Article31. Breinan HA, Martin SD, Hsu HP, Spector M. Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000. 18:781–789.
Article32. Kramer J, Bohrnsen F, Lindner U, Behrens P, Schlenke P, Rohwedel J. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci. 2006. 63:616–626.
Article33. Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage. 2005. 13:655–664.
Article34. Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994. 76:579–592.
Article35. Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002. 10:199–206.
Article36. Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplan-tation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004. 13:595–600.
Article37. Im GI, Kim DY, Shin JH, Hyun CW, Cho WH. Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Joint Surg Br. 2001. 83:289–294.
Article38. Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006. 12:3405–3416.
Article39. Hui JH, Chen F, Thambyah A, Lee EH. Treatment of chondral lesions in advanced osteochondritis dissecans: a comparative study of the efficacy of chondrocytes, mesenchymal stem cells, periosteal graft, and mosaicplasty (osteochondral autograft) in animal models. J Pediatr Orthop. 2004. 24:427–433.40. Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, Ohgushi H, Wakitani S, Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007. 15:226–231.
Article41. Cui JH, Park SR, Park K, Choi BH, Min BH. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 2007. 13:351–360.
Article42. Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007. 23:178–187.
Article43. Lee KB, Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects-a porcine model. Stem Cells. 2007. 25:2964–2971.
Article44. Chen WH, Lai MT, Wu AT, Wu CC, Gelovani JG, Lin CT, Hung SC, Chiu WT, Deng WP. In vitro stage-specific chondrogenesis of mesenchymal stem cells committed to chondrocytes. Arthritis Rheum. 2009. 60:450–459.
Article45. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.
Article46. Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003. 48:418–429.
Article47. Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005. 13:845–853.
Article48. Nathan S, Das De S, Thambyah A, Fen C, Goh J, Lee EH. Cell-based therapy in the repair of osteochondral defects: a novel use for adipose tissue. Tissue Eng. 2003. 9:733–744.
Article49. Recklies AD, Baillargeon L, White C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998. 41:997–1006.
Article50. Fife RS, Caterson B, Myers SL. Identification of link proteins in canine synovial cell cultures and canine articular cartilage. J Cell Biol. 1985. 100:1050–1055.
Article51. Hamerman D, Smith C, Keiser HD, Craig R. Glycosaminoglycans produced by human synovial cell cultures. Coll Relat Res. 1982. 2:313–329.
Article52. Pei M, He F, Boyce BM, Kish VL. Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthritis Cartilage. 2009. 17:714–722.
Article53. Matsumoto T, Kubo S, Meszaros LB, Corsi KA, Cooper GM, Li G, Usas A, Osawa A, Fu FH, Huard J. The influence of sex on the chondrogenic potential of muscle-derived stem cells: implications for cartilage regeneration and repair. Arthritis Rheum. 2008. 58:3809–3819.
Article54. Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, Cheng L, Elisseeff J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A. 2008. 105:20641–20646.
Article55. Hoben GM, Willard VP, Athanasiou KA. Fibrochondrogenesis of hESCs: growth factor combinations and cocultures. Stem Cells Dev. 2009. 18:283–292.
Article56. Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999. 20:45–53.
Article57. Yu J, Du KT, Fang Q, Gu Y, Mihardja SS, Sievers RE, Wu JC, Lee RJ. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials. 2010. 31:7012–7020.
Article58. Salinas CN, Cole BB, Kasko AM, Anseth KS. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007. 13:1025–1034.
Article59. Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 2005. 24:208–218.
Article60. DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000. 8:309–334.
Article61. Tavella S, Bellese G, Castagnola P, Martin I, Piccini D, Doliana R, Colombatti A, Cancedda R, Tacchetti C. Regulated expression of fibronectin, laminin and related integrin receptors during the early chondrocyte differentiation. J Cell Sci. 1997. 110(Pt 18):2261–2270.
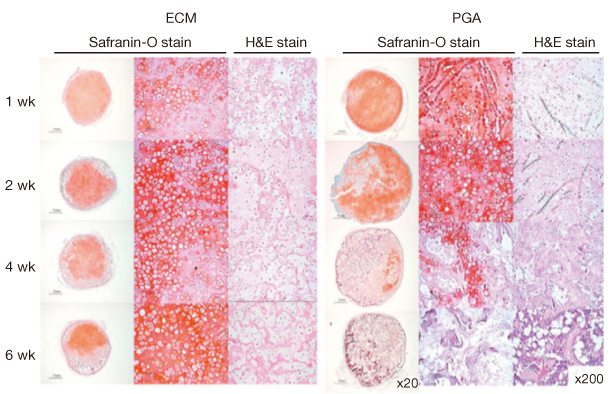
Article62. Jin CZ, Choi BH, Park SR, Min BH. Cartilage engineering using cell-derived extracellular matrix scaffold in vitro. J Biomed Mater Res A. 2010. 92:1567–1577.63. Choi KH, Choi BH, Park SR, Kim BJ, Min BH. The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials. 2010. 31:5355–5365.
Article64. Mobasheri A, Carter SD, Martín-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002. 26:1–18.
Article65. Williams GM, Klisch SM, Sah RL. Bioengineering cartilage growth, maturation, and form. Pediatr Res. 2008. 63:527–534.
Article66. Park K, Cho KJ, Kim JJ, Kim IH, Han DK. Functional PLGA scaffolds for chondrogenesis of bone-marrow-derived mesenchymal stem cells. Macromol Biosci. 2009. 9:221–229.
Article67. Lee HJ, Choi BH, Min BH, Park SR. Low-intensity ultrasound inhibits apoptosis and enhances viability of human mesenchymal stem cells in three-dimensional alginate culture during chondrogenic differentiation. Tissue Eng. 2007. 13:1049–1057.
Article68. Koay EJ, Athanasiou KA. Development of serum-free, chemically defined conditions for human embryonic stem cell-derived fibrochondrogenesis. Tissue Eng Part A. 2009. 15:2249–2257.
Article69. Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001. 268:189–200.
Article70. Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010. 16:159–168.
Article71. Haider HK, Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res. 2010. 3:89–102.
Article