J Korean Neurosurg Soc.
2013 Feb;53(2):65-71. 10.3340/jkns.2013.53.2.65.
Rat Peripheral Nerve Regeneration Using Nerve Guidance Channel by Porcine Small Intestinal Submucosa
- Affiliations
-
- 1Department of Neurosurgery, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea. yangjiho1963@gmail.com
- KMID: 2190680
- DOI: http://doi.org/10.3340/jkns.2013.53.2.65
Abstract
OBJECTIVE
In order to develop a novel nerve guidance channel using porcine small intestinal submucosa (SIS) for nerve regeneration, we investigated the possibility of SIS, a tissue consisting of acellular collagen material without cellular immunogenicity, and containing many kinds of growth factors, as a natural material with a new bioactive functionality.
METHODS
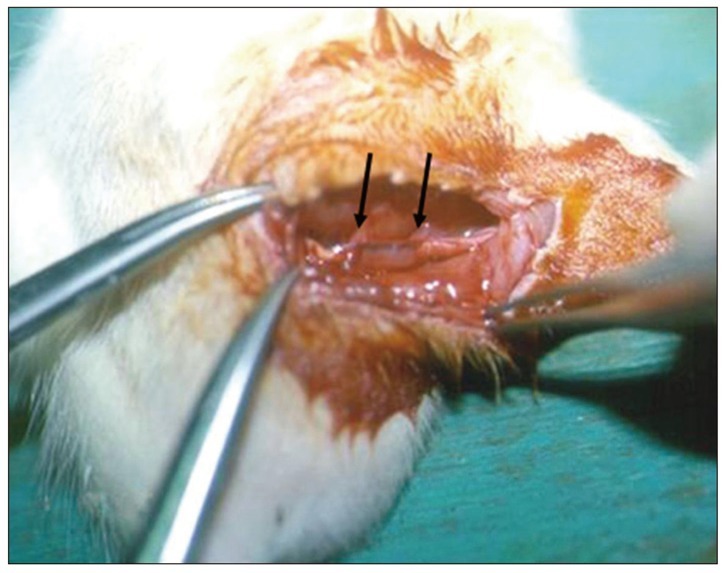
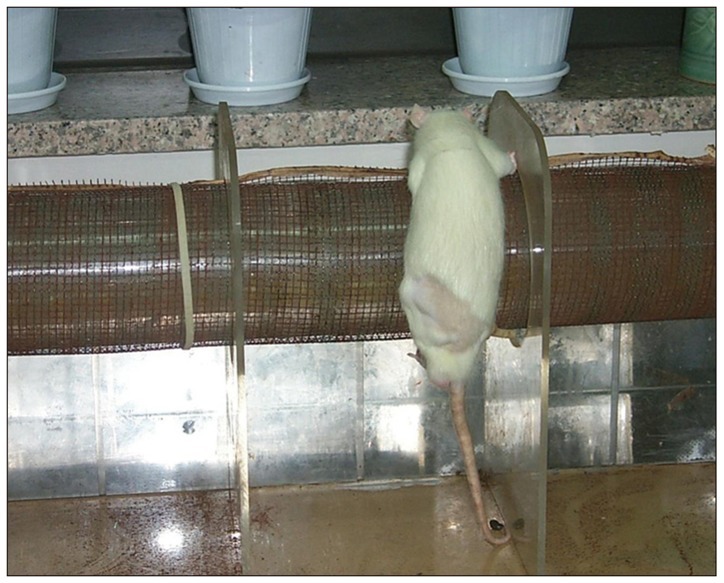
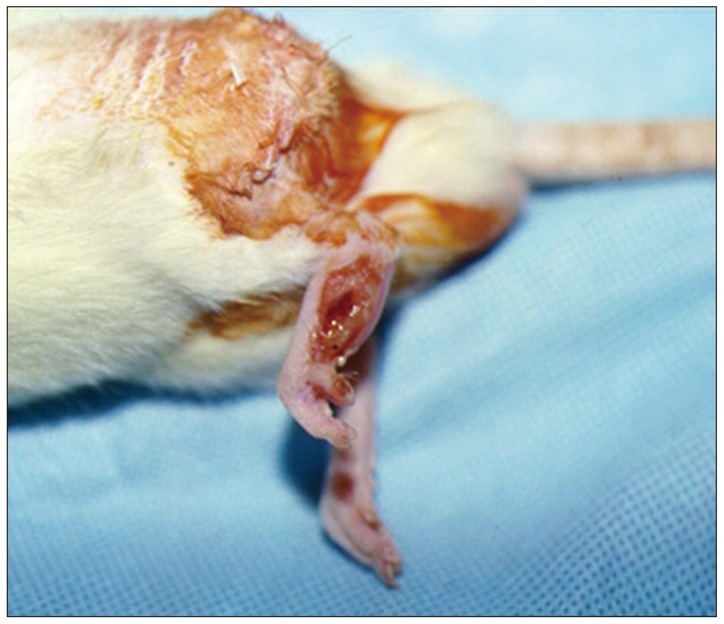
Left sciatic nerves were cut 5 mm in length, in 14 Sprague-Dawley rats. Grafts between the cut nerve ends were performed with a silicone tube (Silicon group, n=7) and rolled porcine SIS (SIS group, n=7). All rats underwent a motor function test and an electromyography (EMG) study on 4 and 10 weeks after grafting. After last EMG studies, the grafts, including proximal and distal nerve segments, were retrieved for histological analysis.
RESULTS
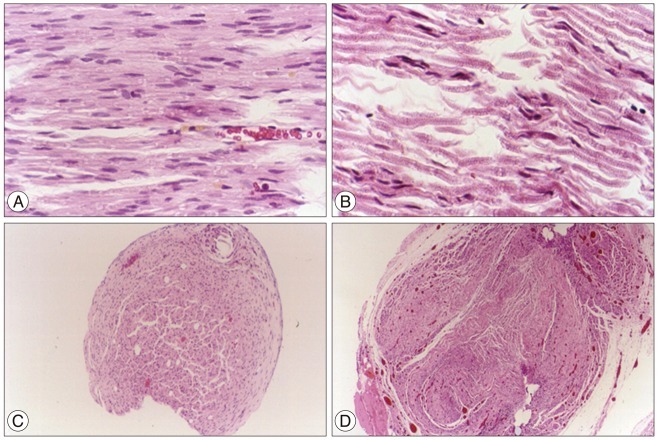
Foot ulcers, due to hypesthesia, were fewer in SIS group than in Silicon group. The run time tests for motor function study were 2.67 seconds in Silicon group and 5.92 seconds in SIS group. Rats in SIS group showed a better EMG response for distal motor latency and amplitude than in Silicon group. Histologically, all grafts contained some axons and myelination. However, the number of axons and the degree of myelination were significantly higher in SIS group than Silicon group.
CONCLUSION
These results show that the porcine SIS was an excellent option as a natural biomaterial for peripheral nerve regeneration since this material contains many kinds of nerve growth factors. Furthermore, it could be used as a biocompatible barrier covering neural tissue.
MeSH Terms
-
Animals
Axons
Collagen
Electromyography
Foot Ulcer
Hypesthesia
Intercellular Signaling Peptides and Proteins
Myelin Sheath
Nerve Growth Factor
Nerve Growth Factors
Nerve Regeneration
Peripheral Nerves
Rats
Rats, Sprague-Dawley
Regeneration
Sciatic Nerve
Silicones
Transplants
Collagen
Intercellular Signaling Peptides and Proteins
Nerve Growth Factor
Nerve Growth Factors
Silicones
Figure
Cited by 1 articles
-
Influencing Factors Analysis of Facial Nerve Function after the Microsurgical Resection of Acoustic Neuroma
WenMing Hong, HongWei Cheng, XiaoJie Wang, ChunGuo Feng
J Korean Neurosurg Soc. 2017;60(2):165-173. doi: 10.3340/jkns.2013.0407.001.
Reference
-
1. Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995; 29:977–985. PMID: 7593041.
Article2. Bejjani GK, Zabramski J. Durasis Study Group. Safety and efficacy of the porcine small intestinal submucosa dural substitute : results of a prospective multicenter study and literature review. J Neurosurg. 2007; 106:1028–1033. PMID: 17564175.
Article3. Borkenhagen M, Stoll RC, Neuenschwander P, Suter UW, Aebischer P. In vivo performance of a new biodegradable polyester urethane system used as a nerve guidance channel. Biomaterials. 1998; 19:2155–2165. PMID: 9884056.
Article4. Caione P, Boldrini R, Salerno A, Nappo SG. Bladder augmentation using acellular collagen biomatrix : a pilot experience in exstrophic patients. Pediatr Surg Int. 2012; 28:421–428. PMID: 22350082.
Article5. Danielsen N, Kerns JM, Holmquist B, Zhao Q, Lundborg G, Kanje M. Predegeneration enhances regeneration into acellular nerve grafts. Brain Res. 1995; 681:105–108. PMID: 7552266.
Article6. den Dunnen WF, van der Lei B, Robinson PH, Holwerda A, Pennings AJ, Schakenraad JM. Biological performance of a degradable poly(lactic acid-epsilon-caprolactone) nerve guide : influence of tube dimensions. J Biomed Mater Res. 1995; 29:757–766. PMID: 7593013.
Article7. Den Dunnen WF, Van der Lei B, Schakenraad JM, Blaauw EH, Stokroos I, Pennings AJ, et al. Long-term evaluation of nerve regeneration in a biodegradable nerve guide. Microsurgery. 1993; 14:508–515. PMID: 8271930.
Article8. Favaro G, Bortolami MC, Cereser S, Doná M, Pastorello A, Callegaro L, et al. Peripheral nerve regeneration through a novel bioresorbable nerve guide. ASAIO Trans. 1990; 36:M291–M294. PMID: 2174684.9. Geoffrion R, Murphy M, Robert M, Birch C, Ross S, Tang S, et al. Vaginal paravaginal repair with porcine small intestine submucosa : midterm outcomes. Female Pelvic Med Reconstr Surg. 2011; 17:174–179. PMID: 22453847.10. Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007; 89:621–630. PMID: 17332112.
Article11. Hadlock T, Elisseeff J, Langer R, Vacanti J, Cheney M. A tissue-engineered conduit for peripheral nerve repair. Arch Otolaryngol Head Neck Surg. 1998; 124:1081–1086. PMID: 9776185.
Article12. Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000; 6:119–127. PMID: 10941207.
Article13. Hadlock T, Sundback C, Koka R, Hunter D, Cheney M, Vacanti J. A novel, biodegradable polymer conduit delivers neurotrophins and promotes nerve regeneration. Laryngoscope. 1999; 109:1412–1416. PMID: 10499046.
Article14. Heath CA, Rutkowski GE. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 1998; 16:163–168. PMID: 9586239.
Article15. Henry EW, Chiu TH, Nyilas E, Brushart TM, Dikkes P, Sidman RL. Nerve regeneration through biodegradable polyester tubes. Exp Neurol. 1985; 90:652–676. PMID: 4065280.
Article16. Kiyotani T, Nakamura T, Shimizu Y, Endo K. Experimental study of nerve regeneration in a biodegradable tube made from collagen and polyglycolic acid. ASAIO J. 1995; 41:M657–M661. PMID: 8573886.
Article17. Kiyotani T, Teramachi M, Takimoto Y, Nakamura T, Shimizu Y, Endo K, et al. Nerve regeneration across a 25-mm gap bridged by a polyglycolic acid-collagen tube : a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 1996; 740:66–74. PMID: 8973799.
Article18. Kropp BP, Rippy MK, Badylak SF, Adams MC, Keating MA, Rink RC, et al. Regenerative urinary bladder augmentation using small intestinal submucosa : urodynamic and histopathologic assessment in long-term canine bladder augmentations. J Urol. 1996; 155:2098–2104. PMID: 8618344.
Article19. Liu Z, Tang R, Zhou Z, Song Z, Wang H, Gu Y. Comparison of two porcine-derived materials for repairing abdominal wall defects in rats. PLoS One. 2011; 6:e20520. PMID: 21637777.
Article20. Lundborg G, Kanje M. Bioartificial nerve grafts. A prototype. Scand J Plast Reconstr Surg Hand Surg. 1996; 30:105–110. PMID: 8815979.21. Maquet V, Martin D, Malgrange B, Franzen R, Schoenen J, Moonen G, et al. Peripheral nerve regeneration using bioresorbable macroporous polylactide scaffolds. J Biomed Mater Res. 2000; 52:639–651. PMID: 11033546.
Article22. Owen K, Wilshaw SP, Homer-Vanniasinkam S, Bojar RA, Berry H, Ingham E. Assessment of the antimicrobial activity of acellular vascular grafts. Eur J Vasc Endovasc Surg. 2012; 43:573–581. PMID: 22340962.
Article23. Roeder R, Wolfe J, Lianakis N, Hinson T, Geddes LA, Obermiller J. Compliance, elastic modulus, and burst pressure of small-intestine submucosa (SIS), small-diameter vascular grafts. J Biomed Mater Res. 1999; 47:65–70. PMID: 10400882.
Article24. Sacks MS, Gloeckner DC. Quantification of the fiber architecture and biaxial mechanical behavior of porcine intestinal submucosa. J Biomed Mater Res. 1999; 46:1–10. PMID: 10357130.
Article25. Tu DD, Seth A, Gil ES, Kaplan DL, Mauney JR, Estrada CR Jr, et al. Evaluation of biomaterials for bladder augmentation using cystometric analyses in various rodent models. J Vis Exp. 2012; in press.
Article26. Utley DS, Lewin SL, Cheng ET, Verity AN, Sierra D, Terris DJ. Brain-derived neurotrophic factor and collagen tubulization enhance functional recovery after peripheral nerve transection and repair. Arch Otolaryngol Head Neck Surg. 1996; 122:407–413. PMID: 8600926.
Article27. Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997; 67:478–491. PMID: 9383707.
Article28. Wang X, Li J, Zhang H, Zhang Y. Evaluation of the small intestinal submucosa covered stent in preventing restenosis after percutaneous transluminal angioplasty in the swine. Eur J Radiol. 2012; 81:e281–e287. PMID: 21704465.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Histological Study of Peripheral Nerve Regeneration in Rats
- Fabrication Techniques of Nerve Guidance Conduits for Nerve Regeneration
- Expression of Caveolin-3 in the Myelin Sheath of Peripheral Nerve
- Estimation of Motor Unit Number According to Severity of Peripheral Nerve Injury in Rat
- Effect of Basic Fibroblast Growth Factor on the Regeneration of the Allografted Sciatic Nerve in Rat