J Adv Prosthodont.
2013 Nov;5(4):402-408. 10.4047/jap.2013.5.4.402.
Effect of magnesium and calcium phosphate coatings on osteoblastic responses to the titanium surface
- Affiliations
-
- 1Dental Research Institute, School of Dentistry, 2nd Stage of Brain Korea 21 Project for School of Dentistry, Chonnam National University, Gwangju, Republic of Korea. cuidezhe@empal.com
- 2R&D Center for Ti and Special Alloys, Gwangju Technopark, Gwangju, Republic of Korea.
- KMID: 2176530
- DOI: http://doi.org/10.4047/jap.2013.5.4.402
Abstract
- PURPOSE
The aim of this study was to evaluate the surface properties and in vitro bioactivity to osteoblasts of magnesium and magnesium-hydroxyapatite coated titanium.
MATERIALS AND METHODS
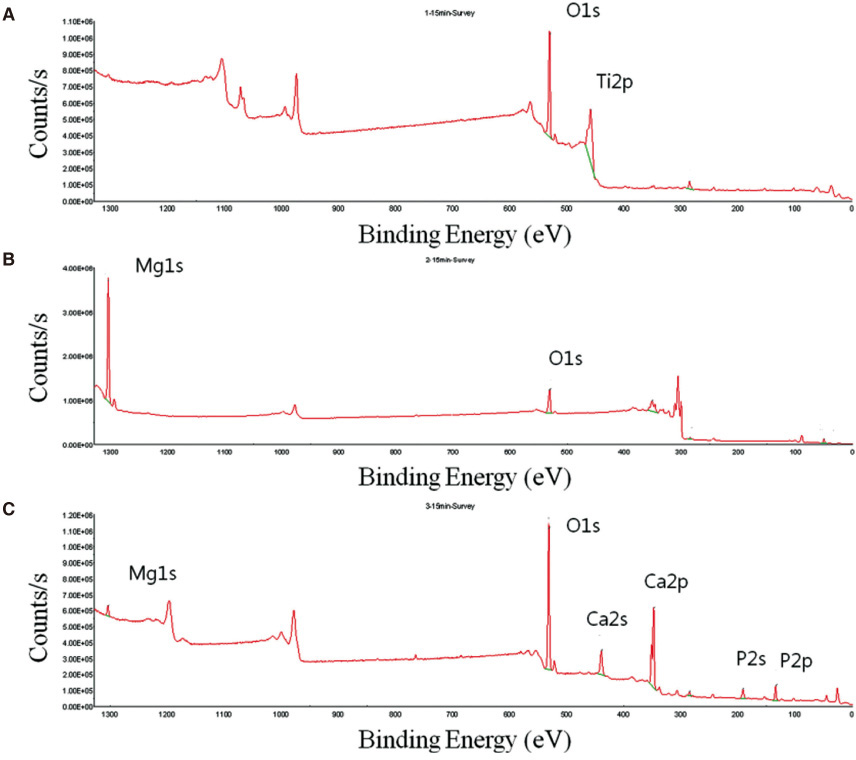
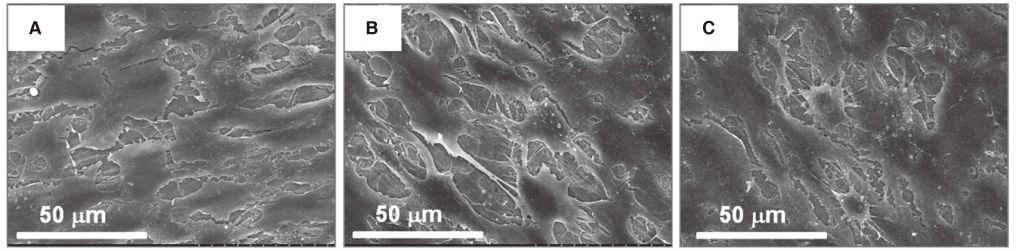
Themagnesium (Mg) and magnesium-hydroxyapatite (Mg-HA) coatings on titanium (Ti) substrates were prepared by radio frequency (RF) and direct current (DC) magnetron sputtering.The samples were divided into non-coated smooth Ti (Ti-S group), Mg coatinggroup (Ti-Mg group), and Mg-HA coating group (Ti-MgHA group).The surface properties were evaluated using scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). The surface roughness was evaluated by atomic force microscopy (AFM). Cell adhesion, cell proliferation and alkaline phosphatase (ALP) activity were evaluated using MC3T3-E1 cells. Reverse transcription polymerase chain reaction (RT-PCR) analysis was performed.
RESULTS
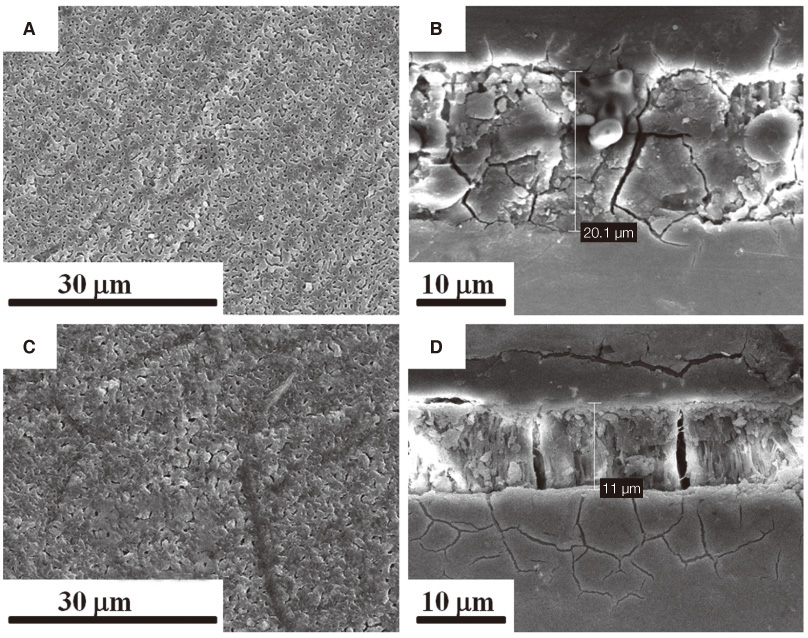
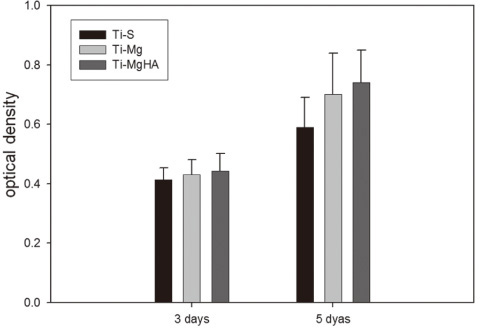
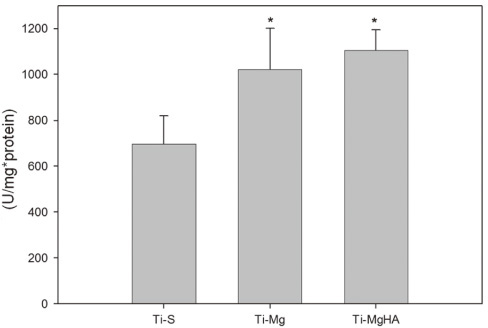
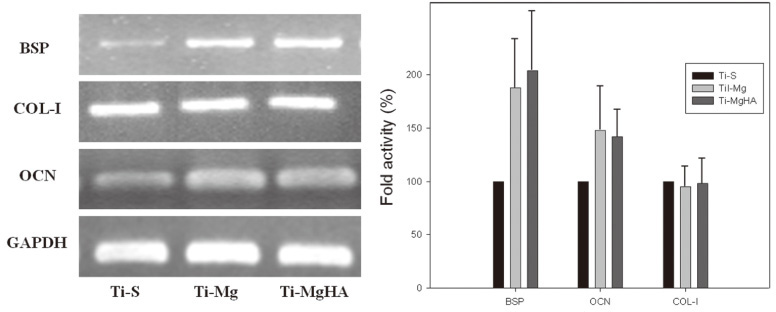
Cross-sectional SEM images showed that Mg and Mg-HA depositionson titanium substrates were performed successfully. The surface roughness appeared to be similaramong the three groups. Ti-MgHA and Ti-Mg group had improved cellular responses with regard to the proliferation, alkaline phosphatase (ALP) activity, and bone-associated markers, such as bone sialoprotein (BSP) and osteocalcin (OCN) mRNA compared to those of Ti-S group. However, the differences between Ti-Mg group and Ti-MgHA group were not significant, in spite of the tendency of higher proliferation, ALP activity and BSP expression in Ti-MgHA group.
CONCLUSION
Mg and Mg-HAcoatings could stimulate the differentiation into osteoblastic MC3T3-E1 cells, potentially contributing to rapid osseointegration.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Biocompatible Materials
Calcium Phosphates
Calcium*
Cell Adhesion
Cell Proliferation
Coated Materials, Biocompatible
Integrin-Binding Sialoprotein
Magnesium*
Microscopy, Atomic Force
Microscopy, Electron, Scanning
Osseointegration
Osteoblasts*
Osteocalcin
Photoelectron Spectroscopy
Polymerase Chain Reaction
Reverse Transcription
RNA, Messenger
Surface Properties
Titanium*
Alkaline Phosphatase
Biocompatible Materials
Calcium
Calcium Phosphates
Coated Materials, Biocompatible
Integrin-Binding Sialoprotein
Magnesium
Osteocalcin
RNA, Messenger
Titanium
Figure
Reference
-
1. Porter AE, Taak P, Hobbs LW, Coathup MJ, Blunn GW, Spector M. Bone bonding to hydroxyapatite and titanium surfaces on femoral stems retrieved from human subjects at autopsy. Biomaterials. 2004; 25:5199–5208.2. Xu L, Pan F, Yu G, Yang L, Zhang E, Yang K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials. 2009; 30:1512–1523.3. Ibasco S, Tamimi F, Meszaros R, Nihouannen DL, Vengallatore S, Harvey E, Barralet JE. Magnesium-sputtered titanium for the formation of bioactive coatings. Acta Biomater. 2009; 5:2338–2347.4. Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006; 27:1728–1734.5. Zreiqat H, Howlett CR, Zannettino A, Evans P, Schulze-Tanzil G, Knabe C, Shakibaei M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J Biomed Mater Res. 2002; 62:175–184.6. Lorenz C, Brunner JG, Kollmannsberger P, Jaafar L, Fabry B, Virtanen S. Effect of surface pre-treatments on biocompatibility of magnesium. Acta Biomater. 2009; 5:2783–2789.7. Na Y, Heo SJ, Kim SK, Koak JY. Implant surface treatments affect gene expression of Runx2, osteogenic key marker. J Adv Prosthodont. 2009; 1:91–96.8. Zreiqat H, Howlett CR, Zannettino A, Evans P, Knabe C, Schulze-Tanzil G, Shakiabei GM. Surface modification of bioceramics affect osteoblastic cells response. Key Eng Mater. 2003; 240-242:707–710.9. Sampaio BV, Göller G, Oktar FN, Valério P, Goes A, Leite MF. Biocompatibility evaluation of three different titanium-hydroxyapatite composites. Key Engin Mater. 2005; 284-286:639–642.10. Hashimoto Y, Kusunoki M, Hatanaka R, Hamano K, Nishikawa H, Hosoi Y, Hontsu S, Nakamura M. Improvement of hydroxyapatite deposition on titanium dental implant using ArF laser ablation: effect on osteoblast biocompatibility in vitro. Adv Sci Technol. 2006; 49:282–289.11. Hulshoff JE, van Dijk K, van der Waerden JP, Wolke JG, Kalk W, Jansen JA. Evaluation of plasma-spray and magnetron-sputter Ca-P-coated implants: an in vivo experiment using rabbits. J Biomed Mater Res. 1996; 31:329–337.12. Barrère F, van der Valk CM, Dalmeijer RA, Meijer G, van Blitterswijk CA, de Groot K, Layrolle P. Osteogenecity of octacalcium phosphate coatings applied on porous metal implants. J Biomed Mater Res A. 2003; 66:779–788.13. Lian JB, Stein GS. The developmental stages of osteoblast growth and differentiation exhibit selective responses of genes to growth factors (TGF beta 1) and hormones (vitamin D and glucocorticoids). J Oral Implantol. 1993; 19:95–105.14. El-Ghannam A, Ducheyne P, Shapiro IM. Porous bioactive glass and hydroxyapatite ceramic affect bone cell function in vitro along different time lines. J Biomed Mater Res. 1997; 36:167–180.15. Wan T, Aoki H, Hikawa J, Lee JH. RF-magnetron sputtering technique for producing hydroxyapatite coating film on various substrates. Biomed Mater Eng. 2007; 17:291–297.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surface characterization of calcium phosphate coating formed on chitosan and alkali-treaDted titanium metal
- Surface analyses of titanium substrate modified by anodization and nanoscale Ca-P deposition
- Cellular Response of Human Bone Marrow Derived Mesenchymal Stem Cells to Titanium Surfaces Implanted with Calcium and Magnesium Ions
- The Experimental Study of Stone Fracture by Shock Wave( I )
- Histomorphometric study of machined titanium implants and calcium phosphate coated titanium implants