J Breast Cancer.
2016 Mar;19(1):76-82. 10.4048/jbc.2016.19.1.76.
Incidence of Febrile Neutropenia in Korean Female Breast Cancer Patients Receiving Preoperative or Postoperative Doxorubicin/Cyclophosphamide Followed by Docetaxel Chemotherapy
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. gmkim77@yuhs.ac
- 2Department of General Surgery, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2176324
- DOI: http://doi.org/10.4048/jbc.2016.19.1.76
Abstract
- PURPOSE
Doxorubicin/cyclophosphamide followed by docetaxel chemotherapy (AC-D) is an intermediate risk factor (incidence of 10%-20%) for febrile neutropenia (FN) in breast cancer. However, the reported incidence of FN while using this regimen was obtained mostly from Western breast cancer patients, with little data available from Asian patients. This study aimed to assess the incidence of FN in Korean breast cancer patients and to describe clinical variables related to FN.
METHODS
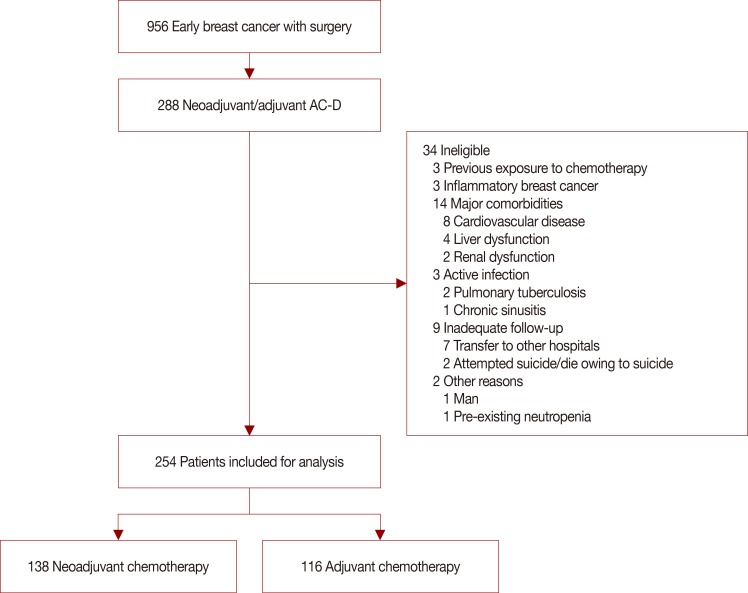
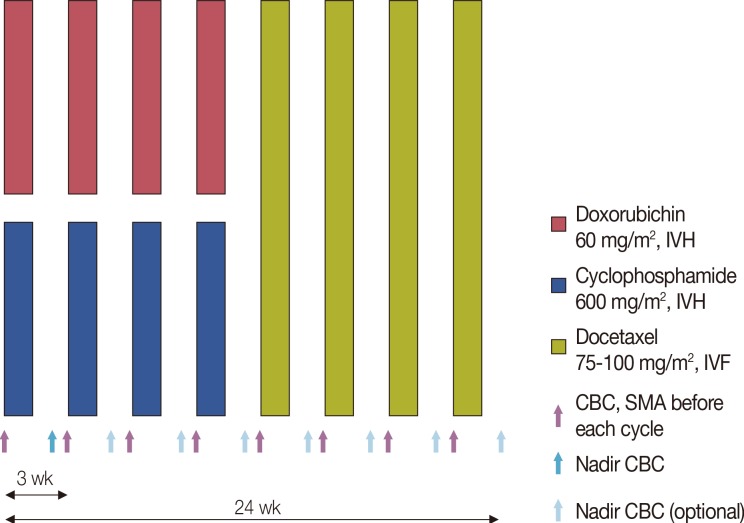
From September 2010 to February 2013, data from the Yonsei Cancer Center registry of breast cancer patients who received neoadjuvant or adjuvant chemotherapy with four cycles of AC-D (60 mg/m2 doxorubicin, 600 mg/m2 cyclophosphamide every 3 weeks for four cycles followed by 75 mg/m2 or 100 mg/m2 docetaxel every 3 weeks for four cycles) were analyzed. The incidence of FN, FN associated complications, dose reduction/delays, and relative dose intensity (RDI) were investigated.
RESULTS
Among the 254 patients reported to the registry, the FN incidence after AC-D chemotherapy was 29.5% (75/254), consisting of 25.2% (64/254) events during AC and 4.7% (12/254) during docetaxel chemotherapy. Dose reductions, delays, and RDI less than 85.0% during AC were observed in 16.5% (42/254), 19.5% (47/254), and 11.0% (28/254) of patients, respectively. Patients with FN events frequently experienced dose reduction/delays, which eventually led to a decreased RDI.
CONCLUSION
The incidence of FN during AC-D neoadjuvant or adjuvant chemotherapy was higher than expected in Korean breast cancer patients. Whether these patients should be classified as a high-risk group for FN warrants future prospective studies.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Clinical utilization of long-acting granulocyte colony-stimulating factor (pegfilgrastim) prophylaxis in breast cancer patients with adjuvant docetaxel-cyclophosphamide chemotherapy
Ye Won Jeon, Seung Taek Lim, Hongki Gwak, Seon Young Park, Juhee Shin, Hye Sug Han, Young Jin Suh
Ann Surg Treat Res. 2021;100(2):59-66. doi: 10.4174/astr.2021.100.2.59.A Phase II Study to Evaluate the Safety and Efficacy of Pegteograstim in Korean Breast Cancer Patients Receiving Dose-Dense Doxorubicin/Cyclophosphamide
Gun Min Kim, Joo Hoon Kim, Ji Heung Kim, Young Up Cho, Seung Il Kim, Seho Park, Hyung Seok Park, Ji Ye Kim, Joohyuk Sohn
Cancer Res Treat. 2019;51(2):812-818. doi: 10.4143/crt.2018.383.
Reference
-
1. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006; 106:2258–2266. PMID: 16575919.
Article2. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007; 25:3158–3167. PMID: 17634496.
Article3. Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998; 90:1205–1211. PMID: 9719081.4. Wildiers H, Reiser M. Relative dose intensity of chemotherapy and its impact on outcomes in patients with early breast cancer or aggressive lymphoma. Crit Rev Oncol Hematol. 2011; 77:221–240. PMID: 20227889.
Article5. Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013; 368:1131–1139. PMID: 23514290.
Article6. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 Update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011; 47:8–32. PMID: 21095116.
Article7. Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006; 24:3187–3205. PMID: 16682719.
Article8. von Minckwitz G, Raab G, Caputo A, Schütte M, Hilfrich J, Blohmer JU, et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol. 2005; 23:2676–2685. PMID: 15837982.
Article9. Vriens BE, Aarts MJ, de Vries B, van Gastel SM, Wals J, Smilde TJ, et al. Doxorubicin/cyclophosphamide with concurrent versus sequential docetaxel as neoadjuvant treatment in patients with breast cancer. Eur J Cancer. 2013; 49:3102–3110. PMID: 23850450.
Article10. Lyman GH, Dale DC, Tomita D, Whittaker S, Crawford J. A retrospective evaluation of chemotherapy dose intensity and supportive care for early-stage breast cancer in a curative setting. Breast Cancer Res Treat. 2013; 139:863–872. PMID: 23771731.
Article11. Eiermann W, Pienkowski T, Crown J, Sadeghi S, Martin M, Chan A, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. J Clin Oncol. 2011; 29:3877–3884. PMID: 21911726.
Article12. Gandara DR, Kawaguchi T, Crowley J, Moon J, Furuse K, Kawahara M, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009; 27:3540–3546. PMID: 19470925.
Article13. Hasegawa Y, Kawaguchi T, Kubo A, Ando M, Shiraishi J, Isa S, et al. Ethnic difference in hematological toxicity in patients with non-small cell lung cancer treated with chemotherapy: a pooled analysis on Asian versus non-Asian in phase II and III clinical trials. J Thorac Oncol. 2011; 6:1881–1888. PMID: 21841503.
Article14. Wang Y, Choueiri TK, Lee JL, Tan MH, Rha SY, North SA, et al. Anti-VEGF therapy in mRCC: differences between Asian and non-Asian patients. Br J Cancer. 2014; 110:1433–1437. PMID: 24548864.
Article15. Huang RS, Kistner EO, Bleibel WK, Shukla SJ, Dolan ME. Effect of population and gender on chemotherapeutic agent-induced cytotoxicity. Mol Cancer Ther. 2007; 6:31–36. PMID: 17237264.
Article16. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes: dealing with the diversity of breast cancer. Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–1747. PMID: 21709140.17. Weycker D, Barron R, Edelsberg J, Kartashov A, Lyman GH. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat. 2012; 133:301–310. PMID: 22270932.
Article18. Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. 2014; 90:190–199. PMID: 24434034.
Article19. Shulman LN, Berry DA, Cirrincione CT, Becker HP, Perez EA, O'Regan R, et al. Comparison of doxorubicin and cyclophosphamide versus single-agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance). J Clin Oncol. 2014; 32:2311–2317. PMID: 24934787.
Article20. Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008; 358:1663–1671. PMID: 18420499.
Article21. Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003; 21:1431–1439. PMID: 12668651.
Article22. Perez EA, Geeraerts L, Suman VJ, Adjei AA, Baron AT, Hatfield AK, et al. A randomized phase II study of sequential docetaxel and doxorubicin/cyclophosphamide in patients with metastatic breast cancer. Ann Oncol. 2002; 13:1225–1235. PMID: 12181246.
Article23. Chan A, Chen C, Chiang J, Tan SH, Ng R. Incidence of febrile neutropenia among early-stage breast cancer patients receiving anthracycline-based chemotherapy. Support Care Cancer. 2012; 20:1525–1532. PMID: 21818641.
Article24. Soo RA, Wang LZ, Ng SS, Chong PY, Yong WP, Lee SC, et al. Distribution of gemcitabine pathway genotypes in ethnic Asians and their association with outcome in non-small cell lung cancer patients. Lung Cancer. 2009; 63:121–127. PMID: 18538445.
Article25. Hor SY, Lee SC, Wong CI, Lim YW, Lim RC, Wang LZ, et al. PXR, CAR and HNF4alpha genotypes and their association with pharmacokinetics and pharmacodynamics of docetaxel and doxorubicin in Asian patients. Pharmacogenomics J. 2008; 8:139–146. PMID: 17876342.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidence of Febrile Neutropenia in Advanced Breast Cancer Patients Receiving Adjuvant Docetaxel-Doxorubicin-Cyclophosphamide Chemotherapy in Korea and Its Impact on Prognosis
- Pegfilgrastim for primary prophylaxis of febrile neutropenia in breast cancer patients undergoing TAC chemotherapy
- Clinical Impact of Primary Prophylactic Pegfilgrastim in Breast Cancer Patients Receiving Adjuvant DocetaxelDoxorubicin-Cyclophosphamide Chemotherapy
- Short Term Effect of Neoadjuvant Therapy with Docetaxel and Adriamycin in Advanced Breast Cancer
- Clinical utilization of long-acting granulocyte colony-stimulating factor (pegfilgrastim) prophylaxis in breast cancer patients with adjuvant docetaxel-cyclophosphamide chemotherapy