J Breast Cancer.
2016 Mar;19(1):34-44. 10.4048/jbc.2016.19.1.34.
Antitumor Effect of IP-10 by Using Two Different Approaches: Live Delivery System and Gene Therapy
- Affiliations
-
- 1Department of Biology, College of Basic Sciences, Damghan Branch, Islamic Azad University, Damghan, Iran.
- 2Department of Immunotherapy and Leishmania Vaccine Research, Pasteur Institute of Iran, Tehran, Iran. s_rafati@yahoo.com
- 3Department of Pathology, Hazrat-e-Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran.
- 4Department of Pharmaceutical Biotechnology, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. el_mohit@yahoo.com, e.mohit@sbmu.ac.ir
- KMID: 2176319
- DOI: http://doi.org/10.4048/jbc.2016.19.1.34
Abstract
- PURPOSE
Immunotherapy is one of the treatment strategies for breast cancer, the most common cancer in women worldwide. In this approach, the patient's immune system is stimulated to attack microscopic tumors and control metastasis. Here, we used interferon γ-induced protein 10 (IP-10), which induces and strengthens antitumor immunity, as an immunotherapeutic agent. We employed Leishmania tarentolae, a nonpathogenic lizard parasite that lacks the ability to persist in mammalian macrophages, was used as a live delivery system for carrying the immunotherapeutic agent. It has been already shown that arginase activity, and consequently, polyamine production, are associated with tumor progression.
METHODS
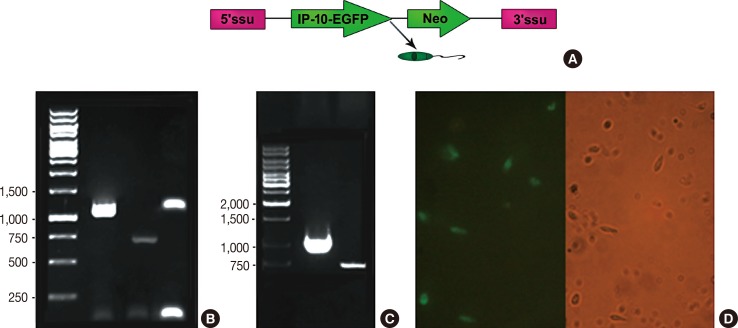
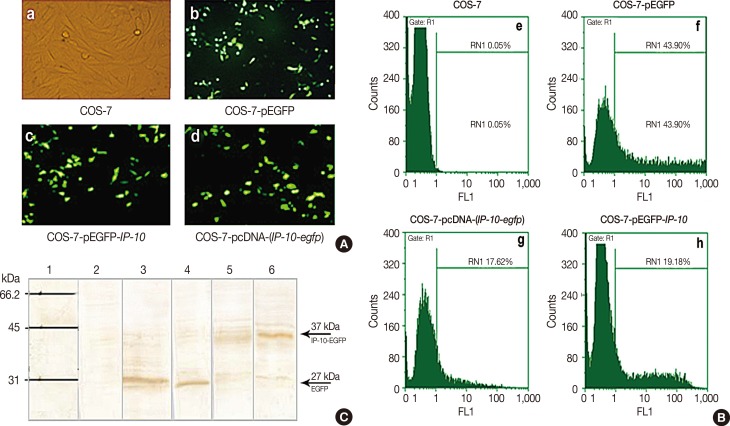
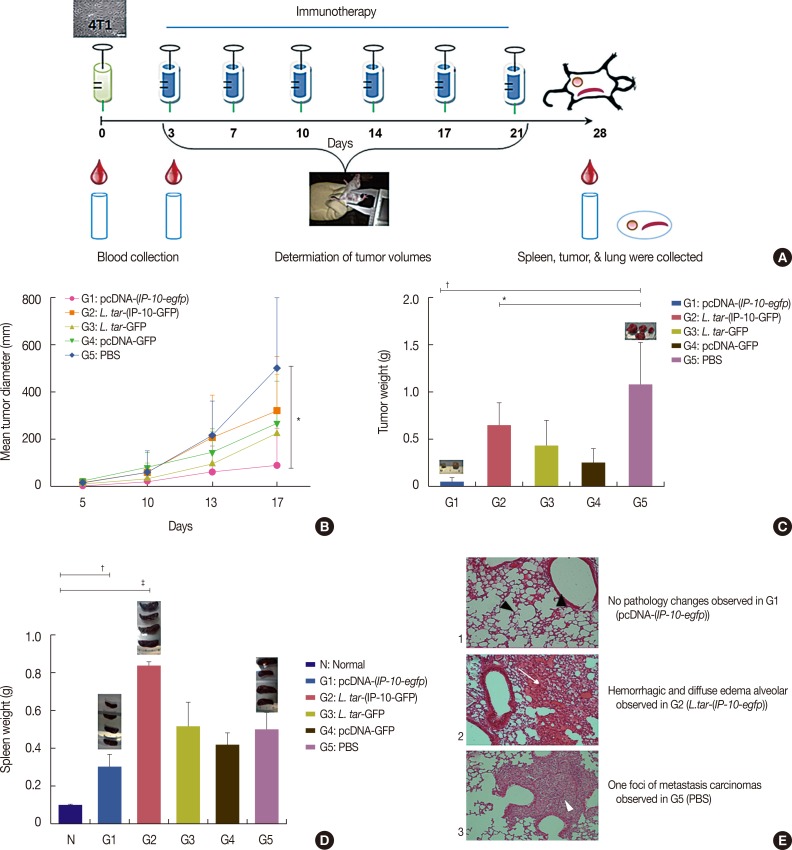
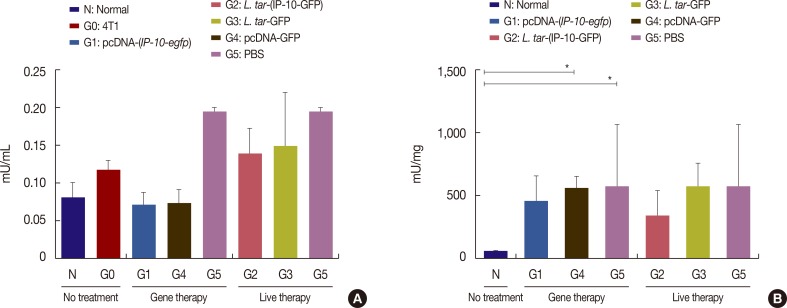
A live delivery system was constructed by stable transfection of pLEXSY plasmid containing the IP-10-enhanced green fluorescent protein (IP-10-egfp) fusion gene into L. tarentolae. Then, the presence of the IP-10-egfp gene and the accurate integration location into the parasite genome were confirmed. The therapeutic efficacy of IP-10 delivered via L. tarentolae and recombinant pcDNA-(IP-10-egfp) plasmid was compared by determining the arginase activity in a mouse 4T1 breast cancer model.
RESULTS
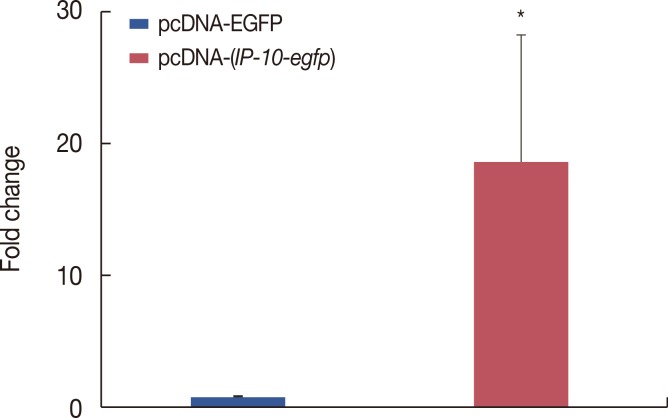
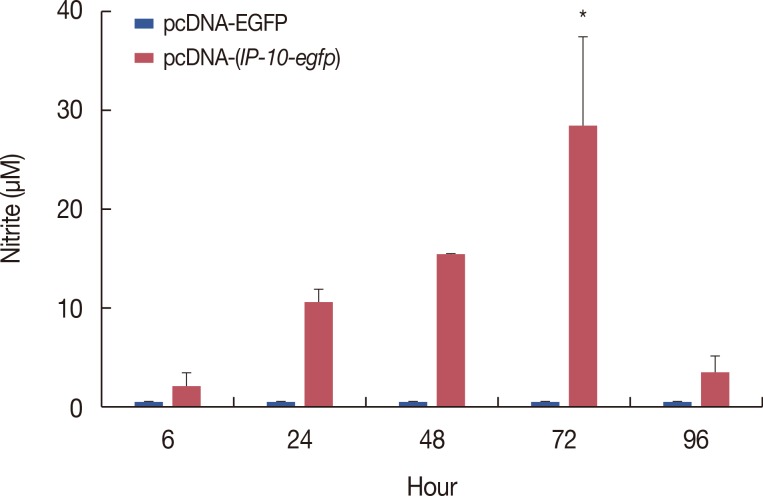
The pcDNA-(IP-10-egfp) group showed a significant reduction in tumor weight and growth. Histological evaluation also revealed that only this group demonstrated inhibition of metastasis to the lung tissue. The arginase activity in the tissue of the pcDNA-(IP-10-egfp) mice significantly decreased in comparison with that in normal mice. No significant difference was observed in arginase activity in the sera of mice receiving other therapeutic strategies.
CONCLUSION
Our data indicates that IP-10 immunotherapy is a promising strategy for breast cancer treatment, as shown in the 4T1-implanted BALB/c mouse model. However, the L. tarentolae-(IP-10-EGFP) live delivery system requires dose modifications to achieve efficacy in the applied regimen (six injections in 3 weeks). Our results indicate that the arginase assay could be a good biomarker to differentiate tumoral tissues from the normal ones.
MeSH Terms
Figure
Reference
-
1. Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009; 20:556–563. PMID: 19073863.
Article2. Hoos A. Evolution of end points for cancer immunotherapy trials. Ann Oncol. 2012; 23(Suppl 8):viii47–viii52. PMID: 22918928.
Article3. Dimberu PM, Leonhardt RM. Cancer immunotherapy takes a multi-faceted approach to kick the immune system into gear. Yale J Biol Med. 2011; 84:371–380. PMID: 22180675.4. Mohit E, Rafati S. Biological delivery approaches for gene therapy: strategies to potentiate efficacy and enhance specificity. Mol Immunol. 2013; 56:599–611. PMID: 23911418.
Article5. Bolhassani A, Zahedifard F. Therapeutic live vaccines as a potential anticancer strategy. Int J Cancer. 2012; 131:1733–1743. PMID: 22610886.
Article6. Breton M, Zhao C, Ouellette M, Tremblay MJ, Papadopoulou B. A recombinant non-pathogenic Leishmania vaccine expressing human immunodeficiency virus 1 (HIV-1) Gag elicits cell-mediated immunity in mice and decreases HIV-1 replication in human tonsillar tissue following exposure to HIV-1 infection. J Gen Virol. 2007; 88(Pt 1):217–225. PMID: 17170454.
Article7. Salehi M, Taheri T, Mohit E, Zahedifard F, Seyed N, Taslimi Y, et al. Recombinant Leishmania tarentolae encoding the HPV type 16 E7 gene in tumor mice model. Immunotherapy. 2012; 4:1107–1120. PMID: 23194361.
Article8. Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007; 117:1155–1166. PMID: 17476345.
Article9. Rodríguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008; 222:180–191. PMID: 18364002.
Article10. Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009; 158:638–651. PMID: 19764983.
Article11. del Ara RM, González-Polo RA, Caro A, del Amo E, Palomo L, Hernández E, et al. Diagnostic performance of arginase activity in colorectal cancer. Clin Exp Med. 2002; 2:53–57. PMID: 12049190.12. Yang F, Gou M, Deng H, Yi T, Zhong Q, Wei Y, et al. Efficient inhibition of ovarian cancer by recombinant CXC chemokine ligand 10 delivered by novel biodegradable cationic heparin-polyethyleneimine nanogels. Oncol Rep. 2012; 28:668–676. PMID: 22684947.
Article13. Pulaski BA. Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001; Chapter 20:Unit 20.2. PMID: 18432775.14. Mohit E, Bolhassani A, Zahedifard F, Seyed N, Eslamifar A, Taghikhani M, et al. Immunomodulatory effects of IP-10 chemokine along with PEI600-Tat delivery system in DNA vaccination against HPV infections. Mol Immunol. 2013; 53:149–160. PMID: 22926003.
Article15. Bolhassani A, Zahedifard F, Taghikhani M, Rafati S. Enhanced immunogenicity of HPV16E7 accompanied by Gp96 as an adjuvant in two vaccination strategies. Vaccine. 2008; 26:3362–3370. PMID: 18471945.
Article16. Taheri T, Saberi Nik H, Seyed N, Doustdari F, Etemadzadeh MH, Torkashvand F, et al. Generation of stable L. major(+EGFP-LUC) and simultaneous comparison between EGFP and luciferase sensitivity. Exp Parasitol. 2015; 150:44–55. PMID: 25637784.17. Bolhassani A, Taheri T, Taslimi Y, Zamanilui S, Zahedifard F, Seyed N, et al. Fluorescent Leishmania species: development of stable GFP expression and its application for in vitro and in vivo studies. Exp Parasitol. 2011; 127:637–645. PMID: 21187086.
Article18. Kropf P, Fuentes JM, Fähnrich E, Arpa L, Herath S, Weber V, et al. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005; 19:1000–1002. PMID: 15811879.
Article19. Gupta G, Bhattacharjee S, Bhattacharyya S, Bhattacharya P, Adhikari A, Mukherjee A, et al. CXC chemokine-mediated protection against visceral leishmaniasis: involvement of the proinflammatory response. J Infect Dis. 2009; 200:1300–1310. PMID: 19743920.
Article20. Yang X, Chu Y, Wang Y, Zhang R, Xiong S. Targeted in vivo expression of IFN-gamma-inducible protein 10 induces specific antitumor activity. J Leukoc Biol. 2006; 80:1434–1444. PMID: 16980511.21. Xanthopoulos JM, Romano AE, Majumdar SK. Response of mouse breast cancer cells to anastrozole, tamoxifen, and the combination. J Biomed Biotechnol. 2005; 2005:10–19. PMID: 15689634.
Article22. Liu M, Guo S, Stiles JK. The emerging role of CXCL10 in cancer (Review). Oncol Lett. 2011; 2:583–589. PMID: 22848232.
Article23. Tang QQ, Shi W, Lü HM, Peng XC, Wen YJ. Antitumor and antimetastatic activities of plasmid pcDNA3. 1-IP10 complexed with cationic liposome in mice with 4T1 breast cancer. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009; 40:195–198. PMID: 19462888.24. Breton M, Tremblay MJ, Ouellette M, Papadopoulou B. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect Immun. 2005; 73:6372–6382. PMID: 16177308.
Article25. Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH, Gravekamp C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer. 2013; 108:2281–2290. PMID: 23640395.
Article26. Mizbani A, Taheri T, Zahedifard F, Taslimi Y, Azizi H, Azadmanesh K, et al. Recombinant Leishmania tarentolae expressing the A2 virulence gene as a novel candidate vaccine against visceral leishmaniasis. Vaccine. 2009; 28:53–62. PMID: 19818721.
Article27. Di Costanzo L, Pique ME, Christianson DW. Crystal structure of human arginase I complexed with thiosemicarbazide reveals an unusual thiocarbonyl mu-sulfide ligand in the binuclear manganese cluster. J Am Chem Soc. 2007; 129:6388–6389. PMID: 17469833.28. Poschke I, Mao Y, Kiessling R, de Boniface J. Tumor-dependent increase of serum amino acid levels in breast cancer patients has diagnostic potential and correlates with molecular tumor subtypes. J Transl Med. 2013; 11:290. PMID: 24237611.
Article29. Nzoumbou-Boko R, Dethoua M, Gabriel F, Buguet A, Cespuglio R, Courtois P, et al. Serum arginase, a biomarker of treatment efficacy in human African trypanosomiasis. J Clin Microbiol. 2013; 51:2379–2381. PMID: 23554207.
Article30. Kazłowska K, Lin HT, Chang SH, Tsai GJ. In vitro and in vivo anticancer effects of sterol fraction from red algae Porphyra dentata. Evid Based Complement Alternat Med. 2013; 2013:493869. PMID: 24062783.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monitoring Gene Therapy by Radionuclide Approaches
- Induction of Dendritic Cell and Cytokine Gene Expression by In situ Delivery of Flt3 Ligand Plasmid
- Detection of Chemokine Gene Expression Induced by IL-12/IL-2 in Renca Tumor
- Gene Therapy and Cell Transplantation for Alzheimer's Disease and Spinal Cord Injury
- Inner Ear Gene Therapy in Mouse Models of Genetic Hearing Loss