J Breast Cancer.
2014 Dec;17(4):386-392. 10.4048/jbc.2014.17.4.386.
Magnetic Resonance Imaging and Clinicopathological Factors for the Detection of Occult Nipple Involvement in Breast Cancer Patients
- Affiliations
-
- 1Department of Surgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. doctor1978@naver.com
- KMID: 2176131
- DOI: http://doi.org/10.4048/jbc.2014.17.4.386
Abstract
- PURPOSE
Nipple sparing mastectomy provides good cosmetic results and low local recurrence rates for breast cancer patients. However, there is a potential risk of leaving an occult tumor within the nipple, which could lead to cancer relapse and poor prognosis for the patient. The objective of this study was to investigate the occult nipple involvement rate in mastectomy specimens, and to identify preoperative magnetic resonance imaging (MRI) findings and the clinicopathological characteristics of the primary tumor that may correlate with nipple invasion.
METHODS
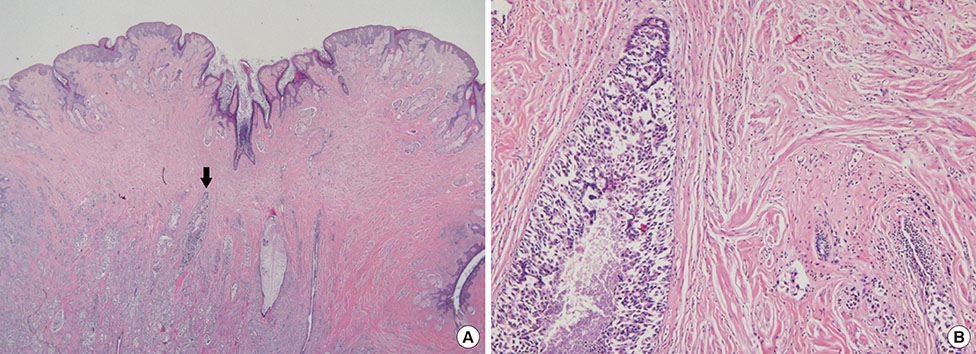
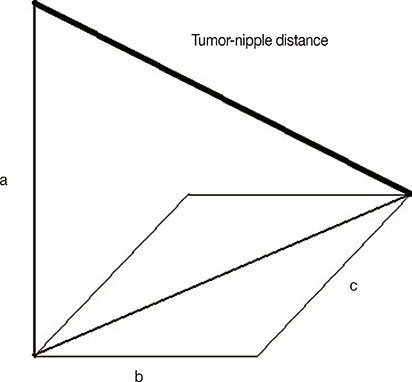
Four hundred sixty-six consecutive mastectomy samples with grossly unremarkable nipples were evaluated. Demographic and clinicopathological data were collected. Nipple involvement was evaluated using serial histological sections. The tumor size and tumor-nipple distance were measured using preoperative MRI images.
RESULTS
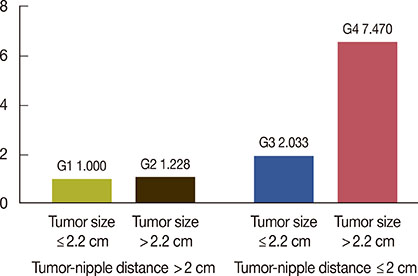
Thirty-six of the 466 therapeutic mastectomy specimens (7.7%) were found to have occult nipple involvement. In univariate analysis, tumor size, tumor-nipple distance, lymph node status, p53 mutation, and lymphovascular invasion (LVI) were found to influence the likelihood of nipple involvement. Multivariate logistic regression analysis, adjusted by lymph node status, p53 mutation, and LVI, showed that tumor size and tumor-nipple distance were predictive factors indicating nipple involvement. With regard to tumor location, only tumors in the central area of the breast showed a significant association with nipple involvement.
CONCLUSION
In this study, a statistically significant association was found between occult nipple involvement and tumor size, tumor-nipple distance, axillary lymph node status, LVI, and p53 mutation. A cutoff point of 2.2 cm for tumor size and 2 cm for tumor-nipple distance could be used as parameters to predict occult nipple involvement.
Keyword
MeSH Terms
Figure
Reference
-
1. Foster RS Jr, Farwell ME, Costanza MC. Breast-conserving surgery for breast cancer: patterns of care in a geographic region and estimation of potential applicability. Ann Surg Oncol. 1995; 2:275–280.
Article2. Curran D, van Dongen JP, Aaronson NK, Kiebert G, Fentiman IS, Mignolet F, et al. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC Trial 10801. The European Organization for Research and Treatment of Cancer (EORTC), Breast Cancer Co-operative Group (BCCG). Eur J Cancer. 1998; 34:307–314.
Article3. Bartelink H, van Dam F, van Dongen J. Psychological effects of breast conserving therapy in comparison with radical mastectomy. Int J Radiat Oncol Biol Phys. 1985; 11:381–385.
Article4. Gerber B, Krause A, Reimer T, Müller H, Küchenmeister I, Makovitzky J, et al. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg. 2003; 238:120–127.
Article5. Sacchini V, Pinotti JA, Barros AC, Luini A, Pluchinotta A, Pinotti M, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg. 2006; 203:704–714.
Article6. Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull. 1962; 30:676–682.
Article7. Bedrosian I, Mick R, Orel SG, Schnall M, Reynolds C, Spitz FR, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003; 98:468–473.
Article8. Schnall MD, Blume J, Bluemke D, Smazal S, Deangelis G, Harms S, et al. MRI detection of multi focal breast carcinoma: report from the International Breast MRI Consortium. J Clin Oncol. 2004; 22:14 Suppl. 504.
Article9. Orel SG, Weinstein SP, Schnall MD, Reynolds CA, Schuchter LM, Fraker DL, et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology. 1999; 212:543–549.
Article10. Smith J, Payne WS, Carney JA. Involvement of the nipple and areola in carcinoma of the breast. Surg Gynecol Obstet. 1976; 143:546–548.11. Andersen JA, Pallesen RM. Spread to the nipple and areola in carcinoma of the breast. Ann Surg. 1979; 189:367–372.
Article12. Wertheim U, Ozzello L. Neoplastic involvement of nipple and skin flap in carcinoma of the breast. Am J Surg Pathol. 1980; 4:543–549.
Article13. Morimoto T, Komaki K, Inui K, Umemoto A, Yamamoto H, Harada K, et al. Involvement of nipple and areola in early breast cancer. Cancer. 1985; 55:2459–2463.
Article14. Santini D, Taffurelli M, Gelli MC, Grassigli A, Giosa F, Marrano D, et al. Neoplastic involvement of nipple-areolar complex in invasive breast cancer. Am J Surg. 1989; 158:399–403.
Article15. Menon RS, van Geel AN. Cancer of the breast with nipple involvement. Br J Cancer. 1989; 59:81–84.
Article16. Verma GR, Kumar A, Joshi K. Nipple involvement in peripheral breast carcinoma: a prospective study. Indian J Cancer. 1997; 34:1–5.17. Vyas JJ, Chinoy RF, Vaidya JS. Prediction of nipple and areola involvement in breast cancer. Eur J Surg Oncol. 1998; 24:15–16.
Article18. Simmons RM, Brennan M, Christos P, King V, Osborne M. Analysis of nipple/areolar involvement with mastectomy: can the areola be preserved? Ann Surg Oncol. 2002; 9:165–168.
Article19. Lazovich D, White E, Thomas DB, Moe RE, Taplin S. Change in the use of breast-conserving surgery in western Washington after the 1990 NIH Consensus Development Conference. Arch Surg. 1997; 132:418–423.
Article20. Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009; 7:193–201.
Article21. Onesti JK, Mangus BE, Helmer SD, Osland JS. Breast cancer tumor size: correlation between magnetic resonance imaging and pathology measurements. Am J Surg. 2008; 196:844–848.
Article22. Schouten van, Boetes C, Bult P, Wobbes T. Magnetic resonance imaging in size assessment of invasive breast carcinoma with an extensive intraductal component. BMC Med Imaging. 2009; 9:5.
Article23. Guidi AJ, Fischer L, Harris JR, Schnitt SJ. Microvessel density and distribution in ductal carcinoma in situ of the breast. J Natl Cancer Inst. 1994; 86:614–619.
Article24. Garcia-Etienne CA, Cody Iii, Disa JJ, Cordeiro P, Sacchini V. Nipple-sparing mastectomy: initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. Breast J. 2009; 15:440–449.
Article25. Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000; 124:966–978.26. Jansson T, Inganäs M, Sjögren S, Norberg T, Lindgren A, Holmberg L, et al. p53 Status predicts survival in breast cancer patients treated with or without postoperative radiotherapy: a novel hypothesis based on clinical findings. J Clin Oncol. 1995; 13:2745–2751.
Article27. Kuske RR, Schuster R, Klein E, Young L, Perez CA, Fineberg B. Radiotherapy and breast reconstruction: clinical results and dosimetry. Int J Radiat Oncol Biol Phys. 1991; 21:339–346.
Article28. Contant CM, van Geel AN, van der Holt B, Griep C, Tjong Joe Wai R, Wiggers T. Morbidity of immediate breast reconstruction (IBR) after mastectomy by a subpectorally placed silicone prosthesis: the adverse effect of radiotherapy. Eur J Surg Oncol. 2000; 26:344–350.
Article29. Vlajcic Z, Zic R, Stanec S, Lambasa S, Petrovecki M, Stanec Z. Nipple-areola complex preservation: predictive factors of neoplastic nipple-areola complex invasion. Ann Plast Surg. 2005; 55:240–244.30. Lester SC. Manual of Surgical Pathology. 2nd ed. Philadelphia: Elsevier Churchill Livingstone;2006.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Magnetic Resonance Findings of Breast Diseases
- Ultrasonographic evaluation of women with pathologic nipple discharge
- The Clinical Significance of Preoperative MRI for Determination of Surgery in Breast Cancer
- A Systematic Review on Radiologists' Knowledge of Breast Cancer Screening
- Nipple Schwannoma: A Case Report and Literature Review on Nipple Mass